Abstract
Glucagon-like peptide 1 (GLP-1) is an incretin hormone responsible for amplification of insulin secretion when nutrients are given orally, as opposed to intravenously, and it retains its insulinotropic activity in patients with type 2 diabetes mellitus. GLP-1-based therapies, such as GLP-1 receptor agonists and inhibitors of dipeptidyl peptidase 4, an enzyme that degrades endogenous GLP-1, have established effectiveness in lowering glucose levels and are routinely used to treat patients with type 2 diabetes. These agents regulate glucose metabolism through multiple mechanisms and have several effects on cardiovascular parameters. These effects, possibly independent of the glucose-lowering activity, include changes in blood pressure, endothelial function, body weight, cardiac metabolism, lipid metabolism, left ventricular function, atherosclerosis, and the response to ischemia–reperfusion injury. Thus, GLP-1-based therapies could potentially target both diabetes and cardiovascular disease. This Review highlights the mechanisms targeted by GLP-1-based therapies, and emphasizes current developments in incretin research that are relevant to cardiovascular risk and disease, as well as treatment with GLP-1 receptor agonists.
Key Points
-
Glucagon-like peptide 1 receptor (GLP-1R) agonists lower blood pressure in patients with type 2 diabetes mellitus
-
GLP-1R agonists have a long-term, weight-reducing effect in the majority of patients with type 2 diabetes
-
Ischemic preconditioning and postconditioning with GLP-1R agonists results in a reduction of ≤50% in the size of myocardial infarctions in animal models, and inhibit myocardial stunning and dysfunction in humans
-
GLP-1R agonists reduce the levels of biomarkers that have been linked to atherosclerotic cardiovascular disease and, therefore, might inhibit atherosclerosis development
-
GLP-1R agonists might improve endothelial dysfunction
-
To date, clinical studies of GLP-1R agonists have shown improvement, rather than impairment, in cardiovascular parameters
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a metabolic disease that affects almost 300 million people worldwide, and this number is expected to approach 450 million by 2030.1,2 In patients with type 2 diabetes, a hereditary predisposition combined with lifestyle and environmental factors results in insulin resistance in skeletal muscle, fatty tissue, and the liver. Loss of or impaired pancreatic β cell function in these individuals results in relative insulin deficiency, which leads to high fasting blood glucose levels. Type 2 diabetes is associated with serious microvascular and macrovascular complications that considerably reduce the quality of life and lifespan of patients.3 In addition, type 2 diabetes is a major risk factor for cardiovascular disease, which has contributed to the increase in incidence and prevalence of the latter.4,5
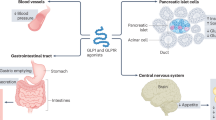
Incretins are gut hormones that lower blood glucose levels by the so-called incretin effect. This effect accounts for the twofold to threefold higher plasma insulin concentrations observed after oral administration of glucose than after an intravenous glucose loading dose that results in similar plasma glucose levels.6 The incretin effect is mediated by glucagon-like peptide 1 (GLP-1), as well as glucose-dependent insulinotropic polypeptide (GIP), secreted from the endocrine cells of the intestinal mucosa in response to food intake. In addition to increasing insulin secretion, GLP-1 also has other effects, such as inhibition of glucagon release from the pancreas, decreased gastrointestinal secretion and motility, and increased satiety and decreased food intake, which all contribute to the antidiabetes actions of GLP-1.7
Clinical studies of GLP-1 and GLP-1 receptor (GLP-1R) agonists have established the effectiveness of these agents as glucose-lowering treatments in patients with type 2 diabetes.8 Studies investigating the therapeutic potential of native GLP-1 have used two different strategies: blocking the actions of dipeptidyl peptidase 4 (DPP-4) with specific inhibitors (such as sitagliptin, vildagliptin, alogliptin, linagliptin, and saxagliptin), which raises plasma levels of endogenous GLP-1 and GIP, and developing DPP-4-resistant GLP-1R agonists, such as liraglutide, exenatide, and lixisenatide.9,10 Exenatide is a synthetic version of exendin-4 (a GLP-1R agonist discovered in 1992 in the saliva of the Gila monster, Heloderma suspectum) that was approved for the treatment of type 2 diabetes in the USA in 2005, and in the EU in 2007. Liraglutide, which is an acylated peptide with 97% sequence homology to native human GLP-1, was approved in the EU in 2009 and in the US in 2010.
Interestingly, an increasing number of studies of GLP-1-based interventions have shown beneficial effects on cardiovascular parameters (such as blood pressure, body weight, and lipid metabolism) that might be independent of improved glycemic control.11 Thus, GLP-1-based therapies potentially target both the diabetes phenotype and cardiovascular risk, although no long-term outcome studies have been completed. This Review provides an overview of published literature and current developments in incretin research of relevance to cardiovascular disease, with an emphasis on treatment strategies using GLP-1R agonists.
Secretion and metabolism of GLP-1
GLP-1 is a cleavage product of proglucagon (Figure 1), secreted from enteroendocrine L cells of the intestinal mucosa in response to nutrient ingestion. GLP-1 levels reach high peaks after having a meal and return to low levels in the fasting state.7 The secreted GLP-1 binds to GLP-1R on pancreatic β cells and stimulates insulin release and synthesis in a strictly glucose-dependent manner.7 Patients with type 2 diabetes often have an impaired GLP-1 response after meals and a decreased sensitivity to GLP-1, but pharmacological levels of GLP-1 (achieved via continuous GLP-1 infusion) normalize fasting blood glucose levels and restore glucose-induced insulin secretion even in patients with long-standing disease.12,13 GLP-1 is also released from neurons in the brain, but the interplay between gut-derived and neuronal GLP-1 is not clear.7
The proglucagon peptide expressed in these three organs is identical, but organ-specific post-translational processing of this propeptide results in various GLP-1 isoforms. Thus, pancreatic GLP-1 differs from that in the gut and brain (although the gut and brain forms of GLP-1 are the same). Numbers below the bars denote the amino acid sequence position. Abbreviations: GLP, glucagon-like peptide; GRPP, glicentin-related pancreatic polypeptide; IP, intervening peptide.
The biologically active form of GLP-1 in humans is GLP-17–36 amide (usually referred to as native GLP-1). The therapeutic potential of native GLP-1 is limited because of its short half-life (1–2 min), owing to its rapid cleavage to GLP-19–36 by DPP-4.14 GLP-19–36 amide does not have insulinotropic properties, but seems to retain glucose-lowering actions. The physiological role of GLP-19–36 amide remains to be established.
GLP-1R
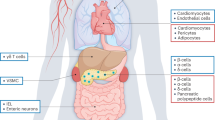
GLP-1R is a member of the glucagon receptor family of G-protein-coupled receptors.15 It binds to GLP-1 with high affinity and has much lower affinity for related peptides, such as GIP and glucagon.16 The tissue distribution of GLP-1R is wide, and its function is not well known at all locations (Box 1). GLP-1R is expressed in many parts of the central nervous system (including in autonomic nuclei that control cardiovascular functions), lung, pancreas. and gastrointestinal tract.17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 Most studies have shown that GLP-1R is present in the kidneys, but not in skeletal muscle, adipose tissue, adipocytes, lymph nodes, spleen, adrenal gland, thymus, or prostate, although its expression in fat tissues, muscle, and liver has been reported, at least in rodents.19,20,21,23,33,34,35 GLP-1R has been identified on calcitonin-producing C cells in mice and rats at much higher levels than in nonhuman primates and humans,36 which might account for the observation in carcinogenicity studies of liraglutide that C-cell tumors were observed in the thyroid tissue of rodents,37 whereas calcitonin levels remain within the normal range despite long-term treatment with high doses of GLP-1 agonists in humans.38 The long-term consequences of sustained GLP-1R activation in the human thyroid, therefore, require further investigation.
Considerable controversy exists over the distribution of GLP-1R in the heart. One study, using an RNAse protection assay, reverse transcriptase PCR, and in situ hybridization techniques, did not find any GLP-1R in the rat heart.19 However, GLP-1R mRNA is clearly present in human hearts, and GLP-1R protein has been found in human coronary artery endothelial cells.33,39 Furthermore, GLP-1R mRNA is found in the mouse heart (endocardium, myocardium, microvascular endothelium, and smooth muscle cells in coronary and mesenteric arteries).18 Studies showing direct actions of GLP-1 on isolated rodent hearts support these reports of expression of GLP-1R in these organs.11,40 By contrast, autoradiography with 135I-GLP-1 did not detect GLP-1R in the human heart.23 GLP-1R expression in the lung and thyroid gland was much higher in rodents than in humans.23 These inconsistent findings could result from problems with the specificity of the antibodies used in each study, the differences in GLP-1R expression among species and, perhaps, handling of the tissues.
Effects of GLP-1 on cardiovascular risk
Native GLP-1 has several effects in the cardiovascular system. This protein can decrease the risk of cardiovascular disease in patients with type 2 diabetes and could also have beneficial effects on cardiovascular risk markers in glucose-tolerant individuals.
Blood pressure and endothelial function
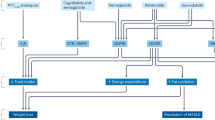
Elevated blood pressure and endothelial dysfunction are established risk factors for cardiovascular disease and are found in about three-quarters of patients with type 2 diabetes.41,42,43 Reducing blood pressure in patients with diabetes and hypertension significantly decreased the risk of myocardial infarction (MI), stroke, microvascular disease, and death in the United Kingdom Prospective Diabetes Study.44 GLP-1 might reduce blood pressure in several ways (Figure 2): improved endothelial function, owing to inhibition of the adverse effects of hyperglycemia;39 increased urine excretion and natriuresis;45 loss of body weight resulting from GLP-1R agonist treatment;46 activation of neural pathways leading to decreased sympathetic nervous system activity; increased insulin production leading to vasodilatation;39,47 and a direct vasodilatory action through GLP-1R stimulation in blood vessels.18,39,47
GLP-1 or GLP-1R agonists reach GLP-1R in target organs directly, but can also reach the area postrema in the brain (via leaks in the blood–brain barrier) and stimulate vagal afferent fibers (via GLP-1R in the gut and the hepatic portal vein). Signaling within the brainstem and hypothalamus results in activation of vagal efferent fibers and sympathetic neurons. These events affect the pulse rate, contractions of the heart, vascular tone, catecholamine secretion from the adrenal medulla, and urine and sodium output in the kidney, thereby modulating blood pressure. Circulating GLP-1 also increases insulin levels via activation of GLP-1R in the pancreas, which dilates blood vessels and lowers glucose levels, inhibiting the endothelial dysfunction caused by hyperglycemia. Abbreviations: AP, area postrema; DMV, dorsal motor nucleus of the vagus; GLP-1, glucagon-like peptide 1, GLP-1R, glucagon-like peptide 1 receptors; NTS, nucleus tractus solitarius; V1, vasopressin receptor.
Preclinical studies
In vitro, native GLP-1 relaxes rat femoral and pulmonary artery rings in a dose-dependent manner, and this action is inhibited by the GLP-1R antagonist exendin9–39.28,48,49 In one study, the removal of endothelium or using a nitric oxide (NO) synthase (NOS) inhibitor did not affect this relaxant effect of GLP-1, suggesting that GLP-1 directly affects smooth muscle cells via GLP-1R.49 In another study, the relaxant effect was eliminated by both the removal of endothelium and use of a NOS inhibitor.28 Relaxation of the rat aorta was dose-dependent, not only with native GLP-1 and exendin-4 treatment, but surprisingly also with GLP-19–36 amide and the GLP-1R antagonist exendin9–39.35 These agents might, therefore, regulate the vascular tone independently of GLP-1R. In rats with streptozotocin-induced or nicotinamide-induced diabetes, vascular responsiveness to acetylcholine or catecholamines was reduced, but was restored in aortic strips from animals that were treated with GLP-1 for 1 month.50 Interestingly, exendin-4 also had a partial normalizing effect on vascular tone.50 Partially preconstricted mouse mesenteric arteries also relaxed when treated with GLP-1 or GLP-19–36 amide, and this effect was independent of glucose levels, but exendin-4 had no vasodilatory effect.18 In this study, GLP-1 and GLP-19–36 amide caused relaxation in the mesenteric arteries, even in GLP-1R-knockout mice, again suggesting a GLP-1R-independent mechanism. Vasodilatation in response to GLP-1 and GLP-19–36 amide was inhibited by NOS inhibitors (L-NNA and L-NAME), suggesting an NO-dependent mechanism.18,51
In vivo, a dose-dependent increase in systolic and diastolic blood pressure and heart rate was observed in rats treated with GLP-1 or exendin-4.52,53 This effect was blocked by pretreatment with exendin9–39; GLP-1 also increased blood pressure in rats pretreated with reserpine, propranolol, or phentolamine, suggesting a GLP-1R-dependent mechanism (rather than a catecholamine-dependent one).52,53,54
Intraportal or intravenous injection of GLP-1 activates afferent vagal nerves in rats,55,56 which might also stimulate ascending neurons of the nucleus of the solitary tract, which express both GLP-1 and GLP-1R.30,56 Intracerebroventricular administration of GLP-1 in rats increased blood pressure and heart rate.52,57,58,59,60 Furthermore, GLP-1-producing neurons project to both the sympathetic preganglionic neurons and the hypothalamus, locally within the brainstem, indicating possible actions on the heart and adrenal medulla and subsequent effects on blood pressure.60 After bilateral vagotomy, intracerebroventricular administration of GLP-1 did not affect blood pressure or heart rate, suggesting that efferent signaling from the brain to the periphery involves the vagus nerve.57 However, intra-arterial injection of a vasopressin V1 receptor antagonist blocked the blood-pressure-stimulating effect of intracerebroventricular GLP-1 in rats, indicating that the effects of this hormone on blood pressure also involve vasopressin released from the posterior pituitary gland.59 Data from two other studies support these findings, by demonstrating elevated levels of vasopressin in response to centrally injected or systemically injected GLP-1.58,59
In salt-sensitive Dahl rats fed a high-salt diet, the increase in blood pressure was attenuated during 14 days of GLP-1 infusion versus that in controls. In the GLP-1-infused rats, sodium excretion and urine flow were increased and renal and cardiac damage were reduced.61 The natriuretic and diuretic response to GLP-1 increased by 13-fold from baseline in innervated kidneys, but was attenuated in denervated kidneys, showing only a threefold increase from baseline.62 The excretion of sodium and urine, therefore, seems to be controlled directly by GLP-1R activation via a neuronal mechanism, and the blood pressure and heart rate are probably modulated by GLP-1 in several ways.
Clinical studies
Clinical data on the effect of GLP-1 on blood pressure and heart rate are conflicting. One study showed that in six patients with type 2 diabetes, 48 h of GLP-1 infusion resulted in a slight, but not statistically significant, reduction in systolic and diastolic blood pressure.63 This treatment had no effect on heart rate.63 In another study, in 15 patients with heart failure, 48 h of GLP-1 infusion led to a slightly increased heart rate and increased diastolic blood pressure, but no change in systolic blood pressure.64 GLP-1 infusion during hyperglycemia in patients with type 2 diabetes and coronary artery disease resulted in increased brachial artery blood flow, but did not affect heart rate or blood pressure, and had no effect in healthy individuals.39 However, GLP-1 infusion increased forearm blood flow during an euglycemic pancreatic clamp procedure (with somatostatin treatment) in individuals without diabetes.47 In a double-blind, placebo-controlled, crossover study in 15 healthy weight men and 16 men with obesity, a 3 h infusion of GLP-1 induced a dose-dependent increase in natriuresis; the greatest effect was seen in men with obesity.45,65
In a post hoc analysis of six randomized, controlled clinical trials comparing the effects of exenatide, placebo, or insulin treatment over 24–52 weeks in 2,171 patients with type 2 diabetes, systolic blood pressure was significantly reduced with exenatide compared with placebo (by 2.8 mmHg) or insulin (by 3.7 mmHg).66 The most pronounced reductions were observed in patients with the highest blood pressures at baseline and were only weakly correlated with weight loss.66 In the DURATION study (1–6 trials)67,68,69 comparing the effect of once-weekly exenatide treatment with other antidiabetes drugs (such as metformin, sulfonylurea and thiazolidinediones), exenatide reduced the systolic blood pressure by 5 mmHg, and the diastolic blood pressure by 2 mmHg.
The LEAD study (1–6 trials)70,71,72,73,74,75 comparing the effects of liraglutide with other antidiabetes drugs showed that treatment with liraglutide reduced the systolic blood pressure by 2–6 mmHg and the diastolic blood pressure by 1–2 mmHg (Figure 3). The reduction in blood pressure was seen after 1–2 weeks of treatment and preceded any loss in body weight. Thus, the early response of blood pressure to liraglutide treatment does not seem to depend on body weight reduction. In patients with obesity but without diabetes, who were treated with liraglutide for 20 weeks during a 2-year extension of the study, the systolic blood pressure was reduced by up to 7.0 mmHg (with 3.0 mg daily treatment of liraglutide) after 1 year and by 4.6 mmHg after 2 years (pooled data from groups of patients treated with 2.4 mg or 3.0 mg of liraglutide).46
These data are from trials that lasted over 6 months with a | liraglutide or b | exenatide.61,62,63,64,65,66,67,68,69,70,113,130 *Significant change from baseline (P <0.05). ‡Patients with obesity but not diabetes mellitus. §No significant difference compared to placebo. ||Placebo-corrected changes. Abbreviations: comb, combination therapy; DPP-4, dipeptidyl peptidase 4; GLP-1R, glucagon-like peptide 1 receptor; Met, metformin, SBP, systolic blood pressure; SU, sulfonylurea; TZD, rosiglitazone.
Heart rate was not reported in most of the above-mentioned clinical trials, but in the LEAD study (trials 1, 4 and 5),72,74,75 heart rate was increased by 2–4 bpm with liraglutide compared with other antidiabetes drugs. However, even a small increase in heart rate accompanying a decrease in blood pressure is troubling, as an increased heart rate is an independent risk factor for cardiac mortality.76,77,78 The mechanism behind the change in heart rate is not known, but might involve increased natriuresis and lowered blood pressure. In one study, patients with obesity but without diabetes were treated with liraglutide and an increase was detected in the heart rate for only the first 30 weeks of treatment.12 The patients' heart rates subsequently returned to basal levels.12 Whether the benefit of the decrease in blood pressure outweighs the harm of the increase in heart rate remains to be determined. Several large cardiovascular outcome trials (LEADER, EXSCEL, ELIXA, REWIND) including up to 9,500 patients with type 2 diabetes are ongoing and are expected to be completed between 2014 and 2019 (Table 1).
Atherosclerosis and inflammatory biomarkers
Atherosclerosis is a complex pathological process associated with multiple inflammatory reactions.79 The formation of an atheroma involves adhesion and infiltration of monocytes into the vascular wall, where inflammation is mediated by cytokines and chemokines. GLP-1 has anti-inflammatory effects and reduces the levels of several biomarkers associated with cardiovascular disease.80
Preclinical studies
One study reported that exendin-4 inhibited monocyte adhesion in the aorta of C57BL/6 (control) mice, as well as markedly reducing the accumulation of monocytes and macrophages in the vascular wall and suppressing the development of atherosclerosis in apolipoprotein E (apoE)-knockout C57BL/6 mice, which have reduced levels of serum apoE and exhibit lipid abnormalities and atherosclerosis, even on a low-cholesterol diet.81,82 Administration of GLP-1 to apoE-knockout mice suppressed the formation of atherosclerotic lesions and macrophage infiltration in the aortic wall, and also decreased foam cell formation.83
Exenatide and liraglutide both reduce serum levels of C-reactive protein (as detected by highly sensitive assays for this inflammatory marker; hsCRP assay), plasminogen activator inhibitor 1 (PAI1), and other inflammatory markers that have been linked to the development of atherosclerotic cardiovascular disease.67,84,85,86 Furthermore, exendin-4 treatment reduced the expression of cytokines, such as tumor necrosis factor (TNF) and CCL2 (also known as monocyte chemotactic protein 1) in activated mouse macrophages, and CD11b—an adhesion molecule—in human monocytes.81 Anti-inflammatory effects of GLP-1 were also reported in human vascular endothelial cells, in which liraglutide increased NO production and suppressed nuclear factor κB activation.
Clinical studies
In 165 patients with type 2 diabetes who were randomly allocated to receive either placebo or liraglutide treatment for 14 weeks, liraglutide significantly reduced the circulating levels of PAI1 and B-type natriuretic peptide (BNP). A dose-dependent, but not statistically significant, reduction in the serum levels of CRP was detected by hsCRP assay; however, no effect was found on the levels of adiponectin, leptin, IL-6, or TNF.87 In a long-term trial, serum total adiponectin levels were increased by 12%, levels of CRP on hsCRP assay were reduced by 61%, and leptin levels were reduced by 16% in 29 patients with type 2 diabetes treated with exenatide for 1 year, compared with the control group of patients with type 2 diabetes treated with insulin glargine.88 In a meta-analysis of six trials of liraglutide in 3,967 patients, BNP was reduced by 12% and CRP levels by 23% (on hsCRP assay) from baseline.85 In 69 patients randomly allocated to receive exenatide or insulin glargine for 1 year, leptin levels were significantly reduced by 14%, hsCRP assay results were significantly reduced by 61%, and total adiponectin levels were increased by 12%, independent of the change in total body fat.88 Another study in patients with type 2 diabetes treated with exenatide once-weekly for 26 weeks also showed a reduction in CRP levels on hsCRP assay.89 Consistently, in the DURATION-2 trial,67 BNP levels were reduced by ∼21%, hsCRP assay results were reduced by ∼24%, PAI1 levels were reduced by ∼8%, and the albumin:creatinine ratio was increased by ∼15% with 26 weeks of treatment with once-weekly exenatide. Interestingly, in another double-blind, placebo-controlled study in 15 patients, treatment with GLP-1 for 48 h had no effect on BNP levels.64 The exact effect of GLP-1-based therapy on inflammation and the development of atherosclerosis needs further elucidation in prospective clinical studies.
Ischemia and ischemia–reperfusion injury
After acute MI, percutaneous coronary intervention (PCI) or thrombolysis are the preferred treatments. However, after opening the blocked vessel, reperfusion injury can occur, causing additional damage to the already ischemic myocardium; this mechanism contributes up to 50% of the final infarct size in animal models.90 Several mechanical and pharmacological interventions have been studied over the years to reduce ischemia–reperfusion injury, using ischemic preconditioning or postconditioning—inducing episodes of nonlethal ischemia and reperfusion, either before or after an episode of lethal myocardial ischemia, respectively—with varying degrees of success.11,18,90
Preclinical studies
In vivo and ex vivo administration of GLP-1 as ischemic preconditioning or postconditioning in five rat models of heart ischemia–reperfusion injury reduced the infarct size by 40–50%.11,40,91,92,93,94 This effect was abolished with the GLP-1R antagonist exendin9–39, and GLP-19–36 amide did not affect the infarct size. Exenatide reduced the infarct size by 39% and prevented the deterioration of cardiac function, as assessed by the recovery of systolic wall thickening and fractional area shortening in pigs.95 Interestingly, one study of liraglutide in a pig model of ischemia–reperfusion injury did not find alterations in the infarct size or hemodynamic parameters in vivo when the drug was given as an ischemic preconditioning agent.96 However, in a similar mouse model, perioperative mortality was reduced, infarction size was decreased (by 27% on day 28 postinfarction), cardiac hypertrophy was reduced, cardiac performance was improved, and the risk of cardiac rupture was decreased after pretreatment with liraglutide for 7 days before MI.97 In a dog model of ischemia–reperfusion injury, in vivo myocardial stunning was shorter and isovolumic left ventricular relaxation was improved after 24 h of ischemic postconditioning with GLP-1.98
Clinical studies
To date, only a few studies have examined the effect of GLP-1 on reperfusion injury in humans. In one study, patients with acute MI and left ventricular ejection fraction (LVEF) <40% received an infusion of GLP-1 for 72 h after treatment with primary angioplasty.98 This treatment significantly improved global and regional cardiac wall motion score indices (a measure of left ventricular function) and LVEF (from 29% ± 2% to 39% ± 2%, P <0.01).99 Similar results were reported in another study, in which 20 patients with normal LVEF and single-vessel coronary artery disease were treated with 1 min of balloon occlusion at the site of the stenosis and randomly allocated to receive GLP-1 or saline infusion.100 The control group had left ventricular stunning and dysfunction 30 min postocclusion (assessed by a reduced rate of change in the left ventricular developed pressure [dP/dt], LVEF, and stroke volume), which were not seen in the GLP-1-treated group, in whom all parameters returned to baseline.100 Furthermore, in a randomized, double-blind, placebo-controlled trial, 105 patients with ST-segment elevation MI and Thrombolysis in Myocardial Infarction flow grade 0 or 1 received exenatide or saline 15 min before and 6 h after primary PCI.101 The infarct size and area at risk were measured using cardiac MRI at admission and 90 days after PCI. The exenatide group had a 15% larger salvage index, corresponding to a 23% smaller infarct size:area at risk ratio, than the placebo group.101
The underlying mechanism of these findings could involve either an antiapoptotic effect of GLP-1, as was demonstrated in isolated mouse HL-1 cardiomyocytes, or a shift in myocardial substrate metabolism from fatty acids to glucose.102 Thus, further human studies using GLP-1R agonists as pharmacological ischemic preconditioning or ischemic postconditioning agents are indicated.
Glucose metabolism
In a healthy heart, 60–90% of ATP is derived from the oxidation of free fatty acids, and the rest from the oxidation of glucose. In heart injury, energy metabolism shifts towards a greater oxidation of glucose than free fatty acids. This shift helps the injured heart, as the oxidation of glucose consumes less oxygen than oxidation of free fatty acids.
Several researchers have, therefore, investigated whether GLP-1 could enhance glucose uptake and thereby facilitate this shift in metabolism, which would be particularly beneficial for patients with diabetes (who have difficulty in switching from free fatty acid to glucose metabolism when energy demands are high). In an open-chest study of the pig heart, GLP-1 administration lowered myocardial lactate and pyruvate levels, and concomitantly increased insulin levels, suggesting that either insulin or GLP-1 increased glucose uptake and, therefore, energy turnover.103 In rat cardiomyocytes, GLP-1 increased acidosis, which was believed to be because of enhanced glycolysis or an alteration in mitochondrial metabolism.104 Dogs with dilated cardiomyopathy had increased myocardial insulin sensitivity and glucose uptake, independent of increased insulin secretion, in response to 48 h of GLP-1 infusion.105,106 Similar data were reported in dogs infused with GLP-19–36 amide.107 GLP-1 also increased glucose uptake in isolated postischemic hearts from rats, independent of insulin.108 In addition, GLP-1 and exendin-4 increased myocardial glucose uptake and oxidation in rat hearts.109 However, in the presence of a fatty acid (oleate), glucose uptake and oxidation were unchanged.109 To date, no studies have examined the effects of GLP-1 on cardiac metabolism in humans.
Left ventricular function
Preclinical studies
GLP-1 does not increase ventricular contraction in isolated cardiomyocytes from rats.104 However, in dogs with pacing-induced (over 28 days) dilated cardiomyopathy treated with GLP-1 for 48 h, LVEF increased from 28% to 38%, and left ventricular dP/dt and stroke volume doubled, whereas left ventricular end-diastolic pressure decreased.106 Similar effects were seen with GLP-19–36 amide.107 Interestingly, plasma levels of GLP-1 were positively correlated with improved diastolic function in elderly men (71 years of age), suggesting that GLP-1 is involved in the regulation of cardiac diastolic function.110
Clinical studies
In one study, 21 patients with or without diabetes and NYHA class III or IV heart failure received a continuous infusion of GLP-1.111 LVEF increased in both groups from 21% to 27%, and 6 min walking distance and quality-of-life scores also improved.111 However, this study was not randomized and the baseline characteristics of the patients were variable. A double-blind, placebo-controlled study in 15 patients with NYHA class II or III heart failure treated for 48 h with GLP-1 showed that this treatment had no effect on the cardiac index, LVEF, tissue Doppler indices, or exercise capacity.64 Nevertheless, in 20 patients with type 2 diabetes undergoing CABG surgery, the control group (not treated with GLP-1) required greater use of inotropic and vasoactive infusions during the initial 48 h postoperation to achieve a similar hemodynamic result to those treated with GLP-1.112
QT interval alterations
Decreases in plasma glucose levels and hypoglycemia have been suggested to prolong the QT interval.113 In a single-dose, placebo-controlled, double-dummy trial, 62 healthy individuals who were initially screened for tolerance to exenatide, were treated with exenatide, placebo (negative control), or moxifloxacin (positive control), and their corrected QT interval (QTC) was measured using Fridericia's method.114 Time-matched QTC was not prolonged with exenatide compared with placebo, but a weak positive correlation was found between plasma concentrations of exenatide and changes (from baseline) in the QTC (slope 0.02, 95% CI 0.01–0.03).114 These results led the FDA to request a further study, using once-weekly exenatide to achieve sustained, high plasma drug concentrations compared with the variable concentrations in the initial QTC study.94 This study included 148 patients from the randomized, open-label, comparator-controlled DURATION-1 trial who were treated with 2 mg of a long-acting release formulation of exenatide once-weekly. The results did not demonstrate a correlation between plasma exenatide concentration and the change in QTC.94 Once-weekly treatment of exenatide was finally approved in the EU in 2011 and is expected to be approved in the US in 2012.
Changes in QTC caused by liraglutide treatment, given at three different doses (0.6 mg, 1.2 mg and 1.8 mg) once a day for 7 days, were assessed in 51 healthy individuals in a randomized, placebo-controlled, double-blind, crossover study.115 Time-matched QTC was not significantly prolonged compared with placebo, and liraglutide concentrations did not correlate with QTC changes.115 Thus, the reported alterations in QTC associated with GLP-1R agonist therapy might not be clinically relevant on the basis of the available evidence. Furthermore, one study in patients undergoing CABG surgery showed fewer occurrences of arrhythmias requiring treatment in GLP-1-treated patients than in controls.116
Body weight
Obesity and being overweight is a common problem for patients with type 2 diabetes, as most therapies for diabetes promote weight gain. Obesity is a major risk factor for cardiovascular disease and increases the total risk of cardiovascular morbidity and mortality in patients with type 2 diabetes; in one study of such patients, intentional weight loss resulted in a 28% reduction in mortality from cardiovascular disease and diabetes.117 In a retrospective analysis of medical data on insulin-treated patients with type 2 diabetes and obesity, treatment with exenatide for 26 weeks significantly reduced the mean body weight by 6.5 kg from baseline, compared with an increase of 2.4 kg from baseline in body weight in the control group, who continued insulin treatment for 26 weeks.86 In 217 patients with type 2 diabetes who completed 3 years of exenatide treatment, body weight was reduced on average by 1.6 kg at week 12 and by 5.3 kg after 3 years, with no apparent plateau.118 The greatest weight loss was seen in patients with elevated serum levels of alanine aminotransferase at baseline or a high BMI, or in those receiving supplementary metformin treatment.118 In a 24-week, double-blind, placebo-controlled study in 232 patients with type 2 diabetes, who had not previously taken any antidiabetes drugs, body weight was reduced by 2.8–3.1 kg in the exenatide-treated group compared with a 1.4 kg reduction in the placebo group.119
Liraglutide treatment for 26 weeks in 533 patients with type 2 diabetes resulted in dose-dependent weight loss (1 kg with 1.2 mg liraglutide and 2 kg with 1.8 mg liraglutide), contrasting with weight gain in the placebo group (0.6 kg).75 Similar results have been reported in other trials of liraglutide (Figure 4).70,71,73 One study assessing the effect of liraglutide on body weight in individuals who have obesity but not diabetes mellitus found that the mean weight loss was significantly greater after 20 weeks of treatment with 1.2–3.0 mg of liraglutide (4.8–7.2 kg mean weight loss) than placebo (2.8 kg mean weight loss) or orlistat (4.1 kg mean weight loss).46 The weight loss associated with liraglutide could be attributed to loss of adipose tissue. In a substudy of the LEAD-2 and LEAD-3 trials, the body fat percentage of liraglutide-treated patients was significantly reduced and dual energy X-ray absorptiometry and CT imaging showed loss of abdominal subcutaneous fat and visceral fatty tissue.120
The included trials lasted over 6 months and investigated a | liraglutide or b | exenatide.46,67,68,69,70,71,72,73,74,75,118,128,129,130,131 *Significant change from baseline (P <0.05). ‡Patients with obesity but not diabetes mellitus. Abbreviations: comb, combination therapy, DPP-4, dipeptidyl peptidase 4; GLP-1R, glucagon-like peptide 1 receptor; Met, metformin, SU, sulfonylurea; TZD, rosiglitazone.
Traditional methods for achieving weight loss, such as low-calorie diet and lifestyle modifications, are often insufficient in patients with diabetes mellitus as any weight lost is quickly regained. However, the weight-reducing effect of GLP-1R agonist treatment seems to be long lasting, at least in clinical trials. One study in patients with obesity but not diabetes who were initially treated with liraglutide for 20 weeks, followed by a 2-year extension, demonstrated a mean weight loss of up to 7.8 kg after 1 year of treatment (P ≤0.0001 compared with placebo or orlistat) and 5.3 kg after 2 years (P <0.001 compared with orlistat).46 However, although the majority of patients do experience a weight reduction with GLP-1R agonist therapy, the response is variable and a few patients do not respond to this treatment. Neither treatment compliance nor dose adjustment have been assessed in these patients.
Lipid metabolism
Several beneficial effects of GLP-1R agonists on lipid profiles have been observed in patients with type 2 diabetes. Exenatide treatment over 3.5 years in 151 patients reduced serum triglyceride levels by 12% (a reduction from baseline of 44 mg/dl), total cholesterol levels by 5% (a reduction of 11 mg/dl), and LDL-cholesterol levels by 6% (a reduction of 12 mg/dl), and increased HDL-cholesterol levels by 24% (a rise of 9 mg/dl).118 However, in 232 patients with type 2 diabetes (who had not previously taken any antidiabetes drugs) treated with exenatide in a double-blind, placebo-controlled study, no significant changes were found in total, HDL-cholesterol, or LDL-cholesterol levels.119 In the LEAD-4 trial75 (which compared liraglutide treatment with placebo in 533 patients with type 2 diabetes), the levels of triglycerides were significantly reduced by 0.4 mmol/l, LDL cholesterol by 0.3 mmol/l (11.58 mg/dl), and free fatty acids by 0.03 mmol/l. These reductions were reported in patients receiving 1.2 mg of liraglutide once a day; although patients receiving 1.8 mg of the drug once a day had similar reductions, the changes were smaller and only the reduction in free fatty acid levels was statistically significant.75 In a meta-analysis of six randomized, controlled phase III trials, including 3,967 patients with type 2 diabetes, liraglutide was compared with other antidiabetes drugs, such as glimepiride, rosiglitazone, insulin glargine, exenatide, and placebo.85 Liraglutide significantly reduced the serum levels of total cholesterol, LDL cholesterol, free fatty acids, and triglycerides compared with baseline, whereas the other drugs and placebo had no significant effect on lipid levels.85
Although some studies suggest that GLP-1 could reduce chylomicron formation after fat-rich meals,121,122 whether or not the changes in cholesterol and fatty-acid levels associated with GLP-1 treatment have any clinical benefit is unknown. Moreover, whether these changes are a direct result of altered lipid metabolism or are an indirect effect of the weight loss remains to be determined.
GLP-1 receptor-independent pathways
The results of some studies suggest that a second GLP-1R, or a GLP-1R-independent pathway, is involved in mediating the cardiovascular effects of GLP-1 treatment, as some effects of GLP-1 on ischemia–reperfusion injury were preserved in GLP-1R-deficient mice.18 The cDNA sequences of the pancreatic and pulmonary GLP-1R differ by only one nucleotide, although the molecular sizes of the two proteins vary.24,33 However, because the lung GLP-1R cDNA sequence is almost completely homologous to that of the pancreatic form of this receptor, the different biochemical features of these tissue-specific forms of GLP-1R are thought to result from post-translational modifications or alternative splicing of the same primary transcript, rather than variations within the coding sequence of the GLP1R gene.24 The biological consequences of the differences in the physiochemical properties of the receptor protein are unknown. Research to identify alternative GLP-1Rs is ongoing.
GLP-19–36 amide seems to share some, but not all, effects of the native peptide, as described in the 'Blood pressure and endothelial function', 'Ischemia and ischemia–reperfusion injury' and 'Left ventricular function' sections above.18,35,93,107 GLP-19–36 amide, unlike native GLP-1, does not have insulinotropic properties and, therefore, its effects might not be mediated through the known GLP-1R pathway, but rather through an unknown GLP-1R, a mechanism unrelated to the glucagon family of receptors, or an unknown mechanism. To date, no studies have reported positive effects of GLP-19–36 amide treatment on the cardiovascular system in humans, and the clinical potential of this peptide has not been established. Currently, no GLP-19–36 amide agonists are commercially available and none is in development.
Future perspectives
Several trials of long-acting release formulations of GLP-1R agonists are currently in progress (Table 2). New formulations optimized for alternative routes of administration, such as oral, transdermal, or nasal treatment, are also under development (Table 2). Other mechanisms of action of these agents are also being explored. In a pig model, GLP-1 improved postresuscitation myocardial microcirculatory dysfunction, and in a rat model of lipopolysaccharide-induced suppression of cardiovascular function, DPP-4-deficient rats had better preservation of cardiovascular function than wild-type rats, indicating that GLP-1R agonists might be used in the treatment of postresuscitation syndrome or septic shock.123,124
The results of meta-analyses have raised concerns that some agents used in the treatment of diabetes might further increase the risk of cardiovascular disease.125,126 Concerns have also been raised about GLP-1R agonists, as long-term outcome trials are lacking.127 Nevertheless, GLP-1R agonists not only benefit patients with hyperglycemia, but also, as stated above, those with additional cardiovascular risk factors. Interestingly, to date, no clinical trials have shown an increased risk of cardiovascular disease in patients treated with GLP-1R agonists. No data are yet available from large prospective cardiovascular outcome trials, but a retrospective analysis of 39,275 patients treated with exenatide reported significantly fewer cardiovascular events in the exenatide-treated group than the control group (HR 0.81, 95% CI 0.68–0.95).128 Four large, randomized cardiovascular outcome trials specifically assessing the effect of incretin-based therapies on the risk of macrovascular complications of diabetes mellitus are in progress (Table 1).
Conclusions
Type 2 diabetes is a major risk factor for cardiovascular disease. GLP-1R agonists are frequently used for the treatment of patients with this disease, because they improve glycemic control to an extent at least equivalent to other treatments for diabetes. In addition, GLP-1R agonists reduce body weight and blood pressure in randomized, controlled trials in patients with type 2 diabetes (as well as in individuals with obesity but without diabetes); such agents might, therefore, also reduce cardiovascular risk. As the decrease in blood pressure precedes weight loss, the antihypertensive effect is probably not dependent on reductions in obesity. Whether the mild, transient increase in heart rate associated with GLP-1R agonist treatment is clinically relevant is unknown and needs further evaluation.
Interestingly, GLP-1R agonists reduce the levels of several inflammatory markers in patients with type 2 diabetes and seem to have beneficial effects on some aspects of the development of atherosclerosis. These effects might delay the onset or progression of atherosclerotic disease. Furthermore, GLP-1R agonists improve lipid profiles and dyslipidemia in patients with type 2 diabetes. Preclinical and clinical studies show that GLP-1 also has beneficial effects on ischemia–reperfusion injury, but these data need to be confirmed in large clinical trials. The reported potential beneficial effects of GLP-1 on cardiovascular morbidity and mortality seem to be clinically relevant, indicating that improvement of long-term cardiovascular risk profiles is possible in patients treated with GLP-1R agonists.
Review criteria
Articles for this Review were identified by searching the PubMed, EMBASE, and Cochrane databases using the following terms: “incretin hormones”, “incretins”, “glucagon-like peptide 1”, “GLP-1-receptor agonists”, “heart disease”, “cardiovascular risk”, “cardiovascular system” and “cardiovascular disease”, without limitation on the article type or language. All relevant full-text papers published between January 1970 and August 2011 were considered. The US clinical trials website was searched for relevant trials, and experts in the field were consulted about relevant topics.
References
International Diabetes Federation. IDF Diabetes Atlas 4th edn (International Diabetes Federation, Brussels, 2009).
Wild, S., Roglic, G., Green, A., Sicree, R. & King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 (2004).
Mata-Cases, M. et al. Incidence of complications and mortality in a type 2 diabetes patient cohort study followed up from diagnosis in a primary healthcare center. Int. J. Clin. Pract. 65, 299–307 (2011).
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 (1998).
Juutilainen, A., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 28, 2901–2907 (2005).
Nauck, M. A. et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 63, 492–498 (1986).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Holst, J. J., Madsbad, S. & Schmitz, O. in Textbook of Diabetes 4th edn (eds Holt, R. I. G., Cockram, C. S., Flyvbjerg, A & Goldstein, B) 478–493 (Wiley–Blackwell Publishing, New Jersey, (2010).
Deacon, C. F. et al. Dipeptidyl peptidase IV resistant analogs of glucagon-like peptide 1 which have extended metabolic stability and improved biological activity. Diabetologia 41, 271–278 (1998).
Deacon, C. F. et al. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44, 1126–1131 (1995).
Bose, A. K., Mocanu, M. M., Carr, R. D., Brand, C. L. & Yellon, D. M. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54, 146–151 (2005).
Nauck, M. A. et al. Preserved incretin effect in type 1 diabetic patients with end-stage nephropathy treated by combined heterotopic pancreas and kidney transplantation. Acta Diabetol. 30, 39–45 (1993).
Vilsbøll, T., Krarup, T., Madsbad, S. & Holst, J. J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 45, 1111–1119 (2002).
Vilsbøll, T., Agersø, H., Krarup, T. & Holst, J. J. Similar elimination rates of glucagon-like peptide 1 in obese type 2 diabetic patients and healthy subjects. J. Clin. Endocrinol. Metab. 88, 220–224 (2003).
Mayo, K. E. et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 (2003).
Fehmann, H. C. et al. Ligand-specificity of the rat GLP-I receptor recombinantly expressed in Chinese hamster ovary (CHO-) cells. Z. Gastroenterol. 32, 203–207 (1994).
Alvarez, E. et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 92, 798–806 (2005).
Ban, K. et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008).
Bullock, B. P., Heller, R. S. & Habener, J. F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137, 2968–2978 (1996).
Campos, R. V., Lee, Y. C. & Drucker, D. J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Egan, J. M., Montrose-Rafizadeh, C., Wang, Y., Bernier, M. & Roth, J. Glucagon-like peptide-1(7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135, 2070–2075 (1994).
Kanse, S. M., Kreymann, B., Ghatei, M. A. & Bloom, S. R. Identification and characterization of glucagon-like peptide 1 7–36 amide-binding sites in the rat brain and lung. FEBS Lett. 241, 209–212 (1988).
Korner, M., Stockli, M., Waser, B. & Reubi, J. C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J. Nucl. Med. 48, 736–743 (2007).
Lankat-Buttgereit, B., Goke, R., Fehmann, H. C., Richter, G. & Goke, B. Molecular cloning of a cDNA encoding for the GLP-1 receptor expressed in rat lung. Exp. Clin. Endocrinol. 102, 341–347 (1994).
Larsen, P. J., Tang-Christensen, M., Holst, J. J. & Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270 (1997).
Merchenthaler, I., Lane, M. & Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403, 261–280 (1999).
Orskov, C. & Poulsen, S. S. Glucagonlike peptide-I-(7–36)-amide receptors only in islets of Langerhans. Autoradiographic survey of extracerebral tissues in rats. Diabetes 40, 1292–1296 (1991).
Richter, G. et al. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. 265, L374–L381 (1993).
Satoh, F. et al. Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe: lack of coupling to either stimulation or inhibition of adenylyl cyclase. Endocrinology 141, 1301–1309 (2000).
Shimizu, I., Hirota, M., Ohboshi, C. & Shima, K. Identification and localization of glucagon-like peptide 1 and its receptor in rat brain. Endocrinology 121, 1076–1082 (1987).
Uttenthal, L. O. & Blazquez, E. Characterization of high-affinity receptors for truncated glucagon-like peptide-1 in rat gastric glands. FEBS Lett. 262, 139–141 (1990).
Uttenthal, L. O., Toledano, A. & Blazquez, E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides 21, 143–146 (1992).
Wei, Y. & Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 358, 219–224 (1995).
Arnes, L., Moreno, P., Nuche-Berenguer, B., Valverde, I. & Villanueva-Penacarrillo, M. L. Effect of exendin-4 treatment upon glucose uptake parameters in rat liver and muscle, in normal and type 2 diabetic state. Regul. Pept. 153, 88–92 (2009).
Green, B. D. et al. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch. Biochem. Biophys. 478, 136–142 (2008).
Bjerre Knudsen, L. et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151, 1473–1486 (2010).
FDA Briefing Materials Table of Contents—Liraglutide. FDA Website [online] (2011).
Hegedüs, L., Moses, AC, Zdravkovic, M, Le Thi, T. & Daniels, GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5,000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J. Clin. Endocrinol. Metab. 96, 853–860 (2011).
Nystrom, T. et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 287, E1209–E1215 (2004).
Sonne, D. P., Engstrom, T. & Treiman, M. Protective effects of GLP-1 analogs exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 146, 243–249 (2008).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Jurado, J. et al. Prevalence of cardiovascular disease and risk factors in a type 2 diabetic population of the North Catalonia diabetes study. J. Am. Acad. Nurse. Pract. 21, 140–148 (2009).
Suwaidi, J. A. et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101, 948–954 (2000).
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317, 703–713 (1998).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond) http://dx.doi.org/10.1038/ijo.2011.158.
Basu, A. et al. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 293, E1289–E1295 (2007).
Golpon, H. A., Puechner, A., Welte, T., Wichert, P. V. & Feddersen, C. O. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul. Pept. 102, 81–86 (2001).
Nystrom, T., Gonon, A. T., Sjoholm, A. & Pernow, J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 125, 173–177 (2005).
Ozyazgan, S., Kutluata, N., Afsar, S., Ozdas, S. B. & Akkan, A. G. Effect of glucagon-like peptide-1(7–36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology 74, 119–126 (2005).
Hansen, L., Hartmann, B., Mineo, H. & Holst, J. J. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul. Pept. 118, 11–18 (2004).
Barragan, J. M., Rodriguez, R. E. & Blazquez, E. Changes in arterial blood pressure and heart rate induced by glucagon- like peptide-1-(7–36) amide in rats. Am. J. Physiol. 266, E459–E466 (1994).
Barragan, J. M., Rodriguez, R. E., Eng, J. & Blazquez, E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul. Pept. 67, 63–68 (1996).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Bucinskaite, V. et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 21, 978–e78 (2009).
Nishizawa, M. et al. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J. Auton. Nerv. Syst. 80, 14–21 (2000).
Barragan, J. M., Eng, J., Rodriguez, R. & Blazquez, E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am. J. Physiol. 277, E784–E791 (1999).
Bojanowska, E. & Stempniak, B. Effects of centrally or systemically injected glucagon-like peptide-1 (7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul. Pept. 91, 75–81 (2000).
Isbil-Buyukcoskun, N. & Gulec, G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul. Pept. 118, 33–38 (2004).
Yamamoto, H. et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest. 110, 43–52 (2002).
Yu, M. et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 21, 1125–1135 (2003).
Moreno, C., Mistry, M. & Roman, R. J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 434, 163–167 (2002).
Toft-Nielsen, M. B., Madsbad, S. & Holst, J. J. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22, 1137–1143 (1999).
Halbirk, M. et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 298, H1096–H1102 (2010).
Gutzwiller, J. P. et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 73, 142–150 (2006).
Okerson, T., Yan, P., Stonehouse, A. & Brodows, R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am. J. Hypertens. 23, 334–339 (2010).
Bergenstal, R. M. et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376, 431–439 (2010).
Diamant, M. et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 375, 2234–2243 (2010).
Drucker, D. J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Buse, J. B. et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374, 39–47 (2009).
Garber, A. et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373, 473–481 (2009).
Marre, M. et al. Liraglutide, a once-daily human GLP-1 analog, added to a sulfonylurea over 26 weeks produces greater improvements in glycemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet. Med. 26, 268–278 (2009).
Nauck, M. et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32, 84–90 (2009).
Russell-Jones, D. et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52, 2046–2055 (2009).
Zinman, B. et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32, 1224–1230 (2009).
Diaz, A., Bourassa, M. G., Guertin, M. C. & Tardif, J. C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur. Heart J. 26, 967–974 (2005).
Jensen, M. T., Marott, J. L., Allin, K. H., Nordestgaard, B. G. & Jensen, G. B. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: The Copenhagen City Heart Study. Eur. J. Cardiovasc. Prev. Rehabil. http://dx.doi.org/10.1177/1741826710394274.
Jouven, X. et al. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 352, 1951–1958 (2005).
Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Hattori, Y. et al. A glucagon-like peptide-1 (GLP-1) analog, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 53, 2256–2263 (2010).
Arakawa, M. et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59, 1030–1037 (2010).
Hirano, T. et al. Incretins directly suppress the development of macrophage-driven atherosclerosis in apolipoprotein E-null mice. Diabetologia 53 (Supl. 1), S72 (2010).
Nagashima, M. et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54, 2649–2659 (2011).
Liu, H., Dear, A. E., Knudsen, L. B. & Simpson, R. W. A long-acting glucagon-like peptide-1 analog attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J. Endocrinol. 201, 59–66 (2009).
Plutzky, J., Garber, A., Falahati, A., Toft, A. D. & Poulter, N. R. The once-daily human GLP-1 analogue, liraglutide, significantly reduces markers of cardiovascular risk in type 2 diabetes: a meta-analysis of six clinical trials [abstract]. Eur. Heart J. 30 (Suppl. 1), 917 (2009).
Viswanathan, P. et al. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr. Pract. 13, 444–450 (2007).
Courreges, J. P. et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analog, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet. Med. 25, 1129–1131 (2008).
Bunck, M. C. et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 33, 1734–1737 (2010).
Diamant, M. et al. Impact of exenatide once weekly and insulin glargine on glucose control and cardiovascular risk f actors in subjects with type 2 diabetes [abstract]. Diabetologia 53 (Suppl. 1), S344 (2010).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Bose, A. K., Mocanu, M. M., Carr, R. D. & Yellon, D. M. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc. Drugs Ther. 19, 9–11 (2005).
Bose, A. K., Mocanu, M. M., Carr, R. D. & Yellon, D. M. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc. Drugs Ther. 21, 253–256 (2007).
Ossum, A., van Deurs, U., Engstrom, T., Jensen, J. S. & Treiman, M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9–36)a in an isolated rat heart. Pharmacol. Res. 60, 411–417 (2009).
Sager, P. et al. Exenatide once weekly did not affect the QTc interval in patients with type 2 diabetes. Presented at the 47th Annual Meeting of the European Association for the Study of Diabetes.
Timmers, L. et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 53, 501–510 (2009).
Kristensen, J. et al. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc. Disord. 9, 31 (2009).
Noyan-Ashraf, M. H. et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 (2009).
Nikolaidis, L. A. et al. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J. Pharmacol. Exp. Ther. 312, 303–308 (2005).
Nikolaidis, LA. et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109, 962–965 (2004).
Read, P. A. et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ. Cardiovasc. Interv. 4, 266–272 (2011).
Lønborg, J. et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehr309.
Ravassa, S., Zudaire, A., Carr, R. D. & Diez, J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 300, H1361–H1372 (2011).
Kavianipour, M. et al. Glucagon-like peptide-1 (7–36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides 24, 569–578 (2003).
Vila Petroff, M. G., Egan, J. M., Wang, X. & Sollott, S. J. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ. Res. 89, 445–452 (2001).
Bhashyam, S. et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ. Heart Fail. 3, 512–521 (2010).
Nikolaidis, L. A. et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110, 955–961 (2004).
Nikolaidis, L. A., Elahi, D., Shen, Y. T. & Shannon, R. P. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 289, H2401–H2408 (2005).
Zhao, T. et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 317, 1106–1113 (2006).
Nguyen, T. D. Oleate controls the effects of GLP-1 and exendin-4 on myocardial glucose utilization and contractile function [abstract]. Eur. Heart J. 37 (Suppl. 1), 937 (2010).
Nathanson, D. et al. Plasma levels of glucagon like peptide-1 associate with diastolic function in elderly men. Diabet. Med. 28, 301–305 (2011).
Sokos, G. G., Nikolaidis, L. A., Mankad, S., Elahi, D. & Shannon, R. P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 12, 694–699 (2006).
Mussig, K. et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am. J. Cardiol. 102, 646–647 (2008).
Heller, S. R. Abnormalities of the electrocardiogram during hypoglycemia: the cause of the dead in bed syndrome? Int. J. Clin. Pract. Suppl. 129, 27–32 (2002).
Linnebjerg, H. et al. A thorough QT study to evaluate the effects of singledose exenatide 10 mug on cardiac repolarization in healthy subjects. Int. J. Clin. Pharmacol. Ther. 49, 594–604 (2011).
Chatterjee, D. J., Khutoryansky, N., Zdravkovic, M., Sprenger, C. R. & Litwin, J. S. Absence of QTc prolongation in a thorough QT study with subcutaneous liraglutide, a once-daily human GLP-1 analog for treatment of type 2 diabetes. J. Clin. Pharmacol. 49, 1353–1362 (2009).
Sokos, G. G. et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 100, 824–829 (2007).
Williamson, D. F. et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 23, 1499–1504 (2000).
Klonoff, D. C. et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24, 275–286 (2008).
Moretto, T. J. et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin. Ther. 30, 1448–1460 (2008).
Jendle, J. et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analog for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 11, 1163–1172 (2009).
Hsieh, J. et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53, 552–561 (2010).
Schwartz, E. A. et al. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 212, 217–222 (2010).
Dokken, B. B. et al. Glucagon-like peptide-1 (GLP-1) attenuates post-resuscitation myocardial microcirculatory dysfunction. Resuscitation 81, 755–760 (2010).
Ku, H. C., Chen, W. P. & Su, M. J. GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats. Naunyn-Schmiedebergs Archives of Pharmacology 382, 463–474 (2010).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 (2007).
Rao, A. D., Kuhadiya, N., Reynolds, K. & Fonseca, V. A. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care 31, 1672–1678 (2008).
Yudkin, J. S., Lehman, R. & Krumholz, H. M. Glucagon-like peptide-1 drugs. Use of GLP-1 analogs needs great caution. BMJ 342, d1478 (2011).
Best, J. H. et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 34, 90–95 (2011).
Blevins, T. et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 96, 1301–1310 (2011).
Buse, J. B. et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27, 2628–2635 (2004).
DeFronzo, R. A. et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28, 1092–1100 (2005).
Kendall, D. M. et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28, 1083–1091 (2005).
Barakat, G. M. et al. Role of glucagon-like peptide-1 and its agonists on early prevention of cardiac remodeling in type 1 diabetic rat hearts. Gen. Physiol. Biophys. 30, 34–44 (2011).
Nakagawa, A. et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 110, 36–43 (2004).
Delgado, E. et al. Glucagon-like peptide-1 binding to rat skeletal muscle. Peptides 16, 225–229 (1995).
Author information
Authors and Affiliations
Contributions
J. Sivertsen, T. Vilsbøll and J. J. Holst contributed to all aspects of the article, including researching data, discussion of content, and writing, reviewing, and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Sivertsen declares no competing interests. J. Rosenmeier declares that she has received grant support from Merck Sharp & Dohme. J. J. Holst declares that he has been a consultant for GlaxoSmithKline, Novo Nordisk, and Zealand Pharmaceuticals. He has also received grants from Merck Sharp & Dohme and Novartis. T. Vilsbøll declares that she has been a consultant for and received honoraria from Amylin, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. She has also received honoraria from Novartis, and grant support from Merck Sharp & Dohme.
Rights and permissions
About this article
Cite this article
Sivertsen, J., Rosenmeier, J., Holst, J. et al. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 9, 209–222 (2012). https://doi.org/10.1038/nrcardio.2011.211
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.211