Abstract
Gastrointestinal bypass surgeries restore metabolic homeostasis in patients with type 2 diabetes and obesity1, but the underlying mechanisms remain elusive. Duodenal-jejunal bypass surgery (DJB), an experimental surgical technique that excludes the duodenum and proximal jejunum from nutrient transit1,2, lowers glucose concentrations in nonobese type 2 diabetic rats2,3,4,5. Given that DJB redirects and enhances nutrient flow into the jejunum and that jejunal nutrient sensing affects feeding6,7, the repositioned jejunum after DJB represents a junction at which nutrients could regulate glucose homeostasis. Here we found that intrajejunal nutrient administration lowered endogenous glucose production in normal rats through a gut-brain-liver network in the presence of basal plasma insulin concentrations. Inhibition of jejunal glucose uptake or formation of long chain fatty acyl-coA negated the metabolic effects of glucose or lipid, respectively, in normal rats, and altered the rapid (2 d) glucose-lowering effect induced by DJB in streptozotocin (STZ)-induced uncontrolled diabetic rats during refeeding. Lastly, in insulin-deficient autoimmune type 1 diabetic rats and STZ-induced diabetic rats, DJB lowered glucose concentrations in 2 d independently of changes in plasma insulin concentrations, food intake and body weight. These data unveil a glucoregulatory role of jejunal nutrient sensing and its relevance in the early improvement of glycemic control after DJB in rat models of uncontrolled diabetes.
Similar content being viewed by others
Main
DJB is an experimental procedure that was developed to investigate the underlying mechanisms responsible for the metabolic benefits of the Roux-en-Y gastric bypass surgery (RYGB). DJB lowers glucose concentrations in nonobese rodents2,3,4,5 and humans8,9 with type 2 diabetes. However, the precise mechanisms that underlie the glucose-lowering effect induced by DJB and its clinical relevance remain elusive.
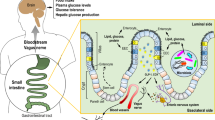
The small intestine activates neuronal and metabolic feedback systems to counteract energy excess and glucose imbalance10,11,12,13,14. Duodenal nutrient sensing lowers food intake in rodents and humans via a gut-brain axis15,16 and lowers endogenous glucose production in rodents via a gut-brain-liver axis17,18. It remains a possibility that other segments of the small intestine could regulate peripheral homeostasis. In fact, intrajejunal lipid infusion lowers food intake in rodents and humans6,7. The underlying mechanisms of jejunal lipid sensing remain unclear, but a gut-brain contribution is implicated19,20, although this has been challenged7. In light of these findings, we propose that jejunal nutrient sensing mechanisms regulate glucose homeostasis and that their identification will begin to unveil mechanisms responsible for the diabetes resolution after DJB.
We first examined whether the jejunum regulates peripheral glucose metabolism in response to intrajejunal infusion of nutrients, such as glucose or lipids (Fig. 1a). Rats were subjected to a pancreatic clamp to obtain basal measurements of glucose metabolism (Fig. 1b and Supplementary Tables 1–3). Given that carbohydrates are a major source of ingested nutrients, we first assessed whether jejunal glucose sensing affects glucose metabolism (Fig. 1b). Intrajejunal glucose administration for 50 min increased the glucose infusion rate and decreased endogenous glucose production (Fig. 1c,d and Supplementary Fig. 1a) during the clamp, whereas plasma insulin concentrations were maintained at basal levels (Supplementary Table 1) and whole-body glucose uptake remained constant (Supplementary Fig. 1b). Whole-body glucose uptake was unaltered by all subsequent intrajejunal treatments (Supplementary Fig. 1b,d,f). To assess whether intrajejunal glucose infusion reduced endogenous glucose production via portal glucose sensing, we infused an equal amount of glucose into the hepatic portal vein (as compared to intrajejunal infusion) for 50 min (Supplementary Fig. 2a). Portal glucose infusion had no effect on whole-body glucose metabolism in the presence of basal insulin concentrations (Supplementary Fig. 2b–e), indicating that the metabolic response of intrajejunal glucose infusion was localized to the jejunum.
(a) Schematic representation of the working hypothesis. (b) Experimental procedure and clamp protocol. NTS, nucleus of the solitary tract; HVAG, hepatic vagotomy; SRIF, somatostatin. i.v., intravenous. (c,d) The glucose infusion rate (c) and the rate of endogenous glucose production (d) during the clamp with intrajejunal glucose, lipid, glucose and phlorizin or lipid and triacsin C (c, **P < 0.01 glucose versus other groups, **P < 0.01 lipid versus other groups; d, *P < 0.05 glucose versus other groups, *P < 0.05 lipid versus other groups). (e–h) The glucose infusion rate (e,g) and the rate of endogenous glucose production (f,h) during the clamp with intrajejunal glucose or lipid infusion alone or in combination with jejunal tetracaine infusion, MK-801 infusion into the NTS or HVAG (e–h, *P < 0.05 versus other groups, **P < 0.01 versus other groups). Values are shown as means ± s.e.m. (one-way analysis of variance (ANOVA)). n = 5 or 6 rats per group.
We next co-infused phlorizin, a sodium-dependent glucose transporter inhibitor21, with glucose intrajejunally to evaluate the necessity of jejunal glucose uptake from the lumen in glucose regulation. Phlorizin negated the ability of jejunal glucose to increase the glucose infusion rate (Fig. 1c) and to reduce endogenous glucose production (Fig. 1d). Plasma glucagon concentrations were unaltered among the groups (for basal: saline: 66 ± 8 pg ml−1; glucose: 55 ± 5 pg ml−1; glucose plus phlorizin: 69 ± 6 pg ml−1; for clamp: saline: 74 ± 15 pg ml−1; glucose: 57 ± 7 pg ml−1; glucose plus phlorizin: 70 ± 12 pg ml−1). Systemic intravenous phlorizin administered at the same dose as jejunal phlorizin did not prevent an intrajejunal glucose infusion from increasing the glucose infusion rate (Supplementary Fig. 3b) and inhibiting endogenous glucose production (Supplementary Fig. 3c,d), thus ruling out any nonintestinal effects of jejunal phlorizin. In addition, intraduodenal or ileal phlorizin infusion (Supplementary Fig. 4a; same dose as jejunal phlorizin) failed to negate the ability of intrajejunal glucose to increase the glucose infusion rate (Supplementary Fig. 4b) and inhibit endogenous glucose production (Supplementary Fig. 4c,d). These data indicate that selective activation of jejunal glucose sensing lowers endogenous glucose production in the presence of basal plasma insulin concentrations.
We next infused Intralipid, a fat emulsion, as a source of long-chain fatty acids (LCFAs) into the jejunum and evaluated the potential glucoregulatory impact (Fig. 1a,b). Intrajejunal lipid infusion for 50 min increased the glucose infusion rate and decreased endogenous glucose production, whereas concurrent infusion of triacsin C, an acyl-CoA synthetase inhibitor, abolished this effect (Fig. 1c,d and Supplementary Fig. 1a), indicating that the formation of jejunal LCFA-CoAs is required for jejunal lipid sensing to lower endogenous glucose production. A gut-brain-liver axis that mediates duodenal lipid sensing to lower endogenous glucose production17 was evaluated for its potential involvement in jejunal nutrient sensing (Fig. 1a). We repeated the intrajejunal glucose or lipid infusion protocol in rats that received: (i) jejunal co-infusion of the anesthetic tetracaine to inhibit jejunal vagal innervation, (ii) MK-801 (NMDA receptor blocker) infusion into the nucleus of the solitary tract or (iii) hepatic branch vagotomy. All treatments fully reversed the ability of intrajejunal glucose or lipid to lower endogenous glucose production (Fig. 1e–h and Supplementary Fig. 1c,e), indicating that jejunal nutrient sensing triggers a neuronal network to lower endogenous glucose production.
Given that jejunal nutrient sensing inhibits endogenous glucose production in normal rats and that DJB redirects and enhances nutrient flow into the remaining distal jejunum and lowers glucose concentrations in nonobese type 2 diabetic rodents2,3,4,5 and humans8,9, we next tested whether jejunal nutrient sensing is required for DJB to lower plasma glucose concentrations in uncontrolled diabetes (Figs. 2 and 3).
(a) Schematic representation of the sham and DJB operation procedure. (b) Experimental procedure. (c) Plasma glucose in normal and STZ-induced diabetic rats before (left) and at 2 d after (right) sham or DJB surgery (***P < 0.001 versus other groups before surgery; **P < 0.01, *P < 0.05 versus other groups at 2 d after surgery). (d) Food intake in normal and STZ-induced diabetic rats before and at 2 d after sham or DJB surgery. (e–g) Fed plasma insulin (e), glucagon (f) and active glucagon-like peptide-1 (GLP-1) (g) before STZ injection, after STZ injection (before surgery) and at 2 d after surgery (e, *P < 0.05 versus other groups; g, *P < 0.05 versus other groups). Values are shown as mean ± s.e.m. (one-way ANOVA). n = 5–8 rats per group.
(a) Schematic representation of the sham and DJB operation procedure and jejunal catheter placement. (b) Experimental procedure. (c) Plasma glucose concentrations during refeeding in STZ-induced diabetic rats given DJB surgery and infused with either intrajejunal vehicle (20% 1,2-proponyldiol, 0.001% DMSO and 0.9% NaCl), phlorizin, triacsin C (Tri-C) or phlorizin plus triacsin C (*P < 0.05 versus all other treatment groups). (d) Accumulated food intake during the refeeding protocol. (e–g) Plasma glucose (e), endogenous glucose production (f), plasma insulin concentration (g) and plasma glucagon concentrations (h) after intrajejunal saline or glucose infusion in nonclamped conditions in STZ-diabetic rats that underwent jejunal and vascular cannulation only (*P < 0.05, **P < 0.01 versus jejunal saline). Values are shown as means ± s.e.m. (Student's t test or one-way ANOVA). n = 5–8 rats per group.
We performed DJB or sham surgery as previously described2 in normal and nonobese STZ-induced diabetic rats (Fig. 2a). We measured the effect of sham or DJB surgery on plasma glucose concentrations at 2 d after surgery to rule out any potential secondary effects on weight loss (Fig. 2b). STZ-injected rats were hyperglycemic (Fig. 2c) and insulin deficient (Fig. 2e) before DJB surgery. Two days after surgery, DJB had a minimal effect on plasma glucose concentrations in nondiabetic rats. Notably, although plasma glucose concentrations were lowered in the STZ-treated, sham-operated (STZ-sham) group at 2 d, DJB further reduced and normalized plasma glucose concentrations compared to sham in STZ-induced diabetic rats (Fig. 2c). This effect was not associated with changes in food intake (Fig. 2d) or body weight (Supplementary Fig. 5), although we did observe a trend of an increase in food intake in STZ-sham rats as compared to other groups. When we matched STZ-sham and STZ-treated, DJB-operated (STZ-DJB) groups for food intake, plasma glucose concentrations remained elevated in the STZ-sham compared to STZ-DJB rats (Fig. 2c), indicating that DJB lowered plasma glucose concentrations independently of changes in food intake. Also, the fact that plasma glucose concentrations were similarly reduced to ∼175 mg dl−1 in pair-fed STZ-treated rats and STZ-sham rats (2 d) (Fig. 2c) indicated that the sham operation may have reduced plasma glucose concentrations to ∼175 mg dl−1 at day 2 as a secondary result of reduced food intake (Fig. 2d). We detected no changes in plasma insulin (Fig. 2e) or glucagon (Fig. 2f) concentrations at 2 d in the STZ-DJB group compared to the STZ-sham group. However, active GLP-1 plasma concentrations were higher in the STZ-DJB group compared to STZ-sham (Fig. 2g). These results indicate that DJB rapidly (in 2 d) lowers plasma glucose concentrations in STZ-induced uncontrolled diabetes, and this glucose lowering is independent of changes in insulin concentrations, food intake and body weight.
We then tested whether jejunal nutrient sensing mediates this early improvement of glycemia induced by DJB in uncontrolled diabetes. We inserted a jejunal catheter at the time of DJB in STZ-treated rats (Fig. 3a) into the same location used for intrajejunal infusion studies in normal rats (Fig. 1a,b). We subsequently performed a fasting-refeeding experiment to activate nutrient-sensing mechanisms while intrajejunally administering phlorizin, triacsin C or both to disrupt jejunal nutrient-sensing mechanisms (Fig. 3b). We monitored plasma glucose for 50 min during refeeding in an attempt to match the duration of intrajejunal nutrient infusion (Fig. 1b). Consistent with the glucose-lowering effect of DJB in STZ-induced diabetic rats (Fig. 2c), the glucose control of nutrient-sensing mechanisms activated by refeeding was intact in STZ-DJB diabetic rats that received intrajejunal vehicle infusion (Fig. 3c), resulting in plasma glucose concentrations (∼120 mg dl−1) comparable to those in STZ-DJB rats at 2 d after surgery (Fig. 2c). In contrast, intrajejunal infusion of phlorizin or triacsin C alone, or in combination disrupted glucose homeostasis during refeeding to a similar degree (Fig. 3c) independently of changes in food intake (Fig. 3d), resulting in plasma glucose concentrations (∼175 mg dl−1) comparable to those in STZ-sham rats at 2 d after surgery (Fig. 2c).
To additionally evaluate whether jejunal nutrient sensing is intact in STZ-DJB rats at 2 d after surgery, we infused glucose into the jejunum of STZ-induced diabetic rats in an unclamped setting. We restricted food intake to 5 g in these STZ-treated rats the night before the experiment to reduce glucose concentrations to ∼180 mg dl−1 (Fig. 3e) in an attempt to match the glucose concentrations of STZ-sham rats 2 days after surgery (Fig. 2c). Intrajejunal glucose infusion for 50 min normalized plasma glucose concentrations (Fig. 3e) and endogenous glucose production (Fig. 3f) independently of changes in plasma insulin and glucagon concentrations (Fig. 3g,h) in these STZ-treated rats. These findings indicate that jejunal nutrient sensing is required for the rapid (2-d) glucose-lowering effect induced by DJB in nonobese, uncontrolled diabetes. The accumulation of LCFA-CoAs in the brain and the liver mediates lipid and glucose sensing to regulate hepatic glucose metabolism14. The fact that intrajejunal infusion of phlorizin together with triacsin C as compared to phlorizin or triacsin C alone did not further disrupt glucose homeostasis in STZ-DJB rats indicates that jejunal glucose- and lipid-sensing mechanisms may converge as they do in the brain and the liver. In addition, in light of the above data and the fact that intrajejunal infusion of phlorizin and triacsin C together did not disrupt glucose homeostasis when normal rats underwent fasting-refeeding (Supplementary Fig. 6a–d), we propose that the rapid glucose-lowering effect of jejunal nutrient sensing in uncontrolled diabetes is revealed apparent after DJB.
To investigate whether the glucose-lowering effect of DJB was transient and whether it would remain in STZ-induced diabetic rats when the confounding glucose-lowering effect induced at 2 d after surgical operation (Fig. 2c) was eliminated, we allowed STZ-sham and STZ-DJB rats to recover for up to 14 d (Fig. 4). Two weeks after surgery, there was no significant difference between the plasma glucose concentrations in nondiabetic DJB-operated and sham-operated rats (Fig. 4a,b). Notably, plasma glucose concentrations in STZ-induced diabetic rats remained normal at 14 d after DJB surgery, whereas the plasma glucose concentrations in STZ-sham rats reached presurgical glucose values (∼400 mg dl−1) (Fig. 4b). The STZ-sham rats became hyperphagic after 14 d compared to the STZ-DJB rats, whose food intake had returned to presurgical levels (Fig. 4c). We observed no changes in body weight between surgical groups after 14 d (Fig. 4c).
(a) Experimental procedure. (b,c) Plasma glucose (b), food intake (c, left) and body weight (c, right) in normal and STZ-induced diabetic rats before and at 14 d after sham or DJB surgery (***P < 0.001 versus other groups before surgery, *P < 0.05, **P < 0.01 versus other groups at 14 d after surgery). (d–f) Plasma glucose (d), food intake (e), and body weight (f) in BB-dp rats before and after (2 d and 6 d) sham or DJB (*P < 0.05, **P < 0.01, ***P < 0.001 versus BB-dp–DJB before surgery, #P < 0.05, ##P < 0.01 versus BB-dp–sham before surgery). (g–i) Fed plasma insulin (g), glucagon (h) and active GLP-1 (i) before and after surgery in BB-dp rats (*P < 0.05 versus before diabetes; g, #P < 0.05 versus BB-dp–sham 6 d after surgery; h, #P < 0.05 versus BB-dp–DJB before surgery). (j) Plasma glucose (left) and endogenous glucose production (right) after intrajejunal saline or glucose infusion in nonclamped conditions in STZ-induced diabetic rats that underwent jejunal and vascular cannulation only (*P < 0.05 versus jejunal saline). Values are shown as mean ± s.e.m. (Student's t test or one-way ANOVA). n = 5–8 rats per group.
To better evaluate whether DJB lowers glucose concentrations in uncontrolled type 1 diabetes, we performed DJB in nonobese, diabetes-prone BioBreeding (BB-dp) rats. BB-dp rats spontaneously develop autoimmune type 1 diabetes and recapitulate many features of human type 1 diabetes, including severe hypoinsulinemia (reviewed in ref. 22). DJB or sham surgery was performed in BB-dp rats 2 d after the onset of hyperglycemia (plasma glucose > 300 mg dl−1), hypoinsulinemia (Fig. 4g) and hyperglucagonemia (Fig. 4h). Plasma glucose remained elevated in control sham-operated rats, comparable to concentrations observed before surgery (Fig. 4d), and the majority of the sham-operated rats did not survive beyond 6 d after surgery. In contrast, DJB lowered plasma glucose concentrations within 2 d of surgery (Fig. 4d), independently of changes in food intake and body weight (Fig. 4e,f), as well as plasma insulin, glucagon and active GLP-1 concentrations (Fig. 4g–i). Of note, the glucose-lowering effect induced by DJB in BB-dp rats was sustained past the 2-d time point and remained 6 d after DJB (Fig. 4d). In contrast to the case at 2 d, when plasma insulin concentrations were similar between sham-operated and DJB-operated rats, at 6 d after surgery plasma insulin concentrations were increased in the rats that received DJB as compared to sham-operated rats (Fig. 4g). Also, sham operation or DJB reduced food intake and body weight to a similar extent in BB-dp rats at both 2 d and 6 d (Fig. 4e,f). Lastly, four out of the five BB-dp–DJB rats survived for at least 3 weeks (data not shown).
To begin evaluating whether jejunal nutrient sensing mediates DJB to lower plasma glucose concentrations in STZ-induced diabetic rats after 14 d (Fig. 4b), we infused glucose into the jejunum of STZ-induced diabetic rats with plasma glucose concentrations (∼325 mg dl−1; Fig. 4j) elevated to a similar extent as in STZ-sham rats at day 14 (Fig. 4b) under nonclamp conditions. Intrajejunal glucose administration for 50 min lowered (but did not normalize) plasma glucose concentrations and endogenous glucose production (Fig. 4j) in these STZ-induced diabetic rats. These findings indicate that jejunal nutrient sensing may partly contribute to the chronic (14-d) glucose-lowering effect induced by DJB in uncontrolled diabetes.
We here report that jejunal nutrient sensing is required for the early improvement of glycemia induced by DJB in nonobese, uncontrolled diabetes. In addition, DJB normalized glucose levels within 2 d of surgery in both insulin-deficient, STZ-induced diabetic rats and in autoimmune type 1 diabetic rats independently of changes in plasma insulin concentrations, food intake and body weight, whereas the glucose-lowering effect induced by DJB in both uncontrolled diabetic models remained beyond 2 d. Bariatric surgery lowers glucose concentrations in type 2 diabetes23,24. However, only a few studies to date have demonstrated that DJB lowers glucose concentrations in nonobese type 2 diabetic rodents2,3,4,5 and humans8,9. We here propose that the redirection and enhancement of nutrient flow into the jejunum that are triggered shortly after a meal mediate the rapid glucose-lowering effect induced by DJB in uncontrolled diabetes. Thus, our current findings indicate a therapeutic potential of gastrointestinal bypass procedures for both type 1 and type 2 diabetes.
Although the underlying mechanisms responsible for the glucose-lowering effect induced by DJB in type 2 and type 1 diabetes remain to be explored, a few sets of observations are worth noting. First, bariatric surgery induces a rapid glucose-lowering effect in type 2 diabetes25,26, whereas DJB also lowers glucose concentrations in nonobese type 2 diabetic rats2,3,4,5 and humans8,9 as well as in nonobese uncontrolled diabetic rats (this study) independent of a reduction in food intake and body weight.
Second, consistent with the fact that jejunal nutrient sensing, which mediates the early improvement (2 d) in glucose homeostasis by DJB in uncontrolled diabetes, is achieved through a reduction in endogenous glucose production (this study), a stomach-sparing proximal intestinal bypass operation (a variant of DJB) has previously been shown to improve glucose homeostasis by a selective reduction in endogenous glucose production in high-fat diet–fed mice28. Similarly in high-fat diet–fed rats, a selective improvement on hepatic insulin sensitivity is seen with RYGB29. Thus, these findings indicate that the inhibition of endogenous glucose production induced by jejunal nutrient sensing mediates the rapid (2 d) glucose-lowering effect induced by DJB in uncontrolled diabetes. However, it is notable that changes in glucose use and/or mechanisms that are activated independently of jejunal nutrient sensing may be responsible for the chronic (14 d) glucose-lowering effect induced by DJB in uncontrolled diabetes. Future studies are warranted to clarify the underlying mechanisms responsible for the acute versus chronic glucose-lowering effect induced by DJB in diabetes. In addition, it would be important to determine the concentration of glucoregulatory hormones at 14 d after DJB in STZ-treated rats. This should begin to shed light on the mechanisms involved in the sustained lowering of plasma glucose concentrations induced by DJB. The relative contribution of jejunal versus portal nutrient sensing in triggering the central nervous system to reduce endogenous glucose production in DJB-diabetic rodents28 also remain to be clarified.
Third, although we documented hypoinsulinemia in both models of uncontrolled diabetes, we still detected insulin in the plasma. This finding indicates that a considerable amount of beta cells may still persist at this stage. Given that plasma insulin concentrations were increased in BB-dp rats at 6 d after DJB, it remains to be assessed whether DJB enhances insulin secretion to lower plasma glucose concentrations in nonobese, uncontrolled diabetes.
Fourth, although RYGB increases plasma GLP-1 concentrations in association with a glucose improvement in obese type 2 diabetes1, DJB reduces endogenous glucose production in high-fat diet–fed mice independent of GLP-1 action28. We here found that at 2 d after surgery DJB normalized glucose concentrations in STZ-induced uncontrolled diabetic rats in association with a rise in plasma GLP-1 concentrations. However, the normalization of glucose concentrations induced by DJB in autoimmune type 1 diabetic rats was independent of changes in GLP-1 concentrations. Thus, the functional relevance of GLP-1 in mediating the rapid glucose-lowering effect induced by DJB in diabetes remains to be clarified.
Last, DJB affects the mixing of bile with nutrients in the duodenum and may consequently alter bile acid metabolism. In fact, the higher serum bile acid levels observed in patients after RYGB are postulated to mediate the improvement of glucose metabolism30. In light of the fact that biliary excretory function is altered in STZ-induced diabetes31, the potential involvement of bile acid metabolism in the ability of DJB to improve glucose homeostasis in uncontrolled diabetic models warrants future investigation.
In summary, we here provide evidence indicating that activation of jejunal nutrient-sensing mechanisms mediates the ability of DJB to rapidly lower glucose concentrations in insulin-deficient, uncontrolled diabetes independently of weight loss. In parallel, DJB normalizes glucose concentrations in autoimmune type 1 diabetic rats. These findings further support the emerging use of gastrointestinal surgery for the treatment of diabetes and unveil sensing mechanisms in the jejunum as potential therapeutic targets for this condition.
Methods
Rats.
The rat protocols were approved by the Institutional Animal Care and Use Committee of the University Health Network. Male Sprague-Dawley rats were obtained from Charles River Laboratories (Montreal, QC), and male diabetes prone BioBreeding (BB-dp) rats were obtained from Biomedical Research Models/Biomere (Baltimore, MD). All rats were maintained on a standard light-dark cycle with access to rat chow and water ad libitum. Uncontrolled diabetes was induced in male Sprague-Dawley rats with a single intraperitoneal injection of streptozotocin (STZ; Sigma; 45mg/kg body weight), administered 5–6 d before sham or DJB surgery. Only rats that were sufficiently hyperglycemic (plasma glucose concentration > 300 mg dl−1) were included in the study.
Surgery.
Rats were subjected to cannulations of the jejunum, duodenum, ileum and the internal jugular vein and carotid artery for infusion and blood sampling during the pancreatic clamp in vivo studies17,32. Separate groups of rats also received cannulation of the hepatic portal vein32, hepatic vagotomy, stereotaxic surgery to implant a bilateral catheter into the nucleus of solitary tract or hepatic branch vagotomy as previously described17,33. Duodenal-jejunal bypass surgery (DJB) was adapted from the duodenal-jejunal exclusion procedure as previously described2 and illustrated in Figure 2a. Sham surgery involved the same procedure as DJB except that all the transections and cuts were sutured back together at the original positions (details in the Supplementary Methods).
Treatments.
The following substances used in the glucose studies were infused through a jejunal catheter (with the exception of phlorizin, which was independently infused into the duodenum and ileum in a subgroup of rats) at a rate of 2 μl min−1 from t = 150 to 200 min: saline; glucose (4 nmol min−1); phlorizin (50 μM); glucose (4 nmol min−1) plus phlorizin (50 μM); tetracaine (0.002 mg min−1); or glucose (4 nmol min−1) plus tetracaine (0.002 mg min−1).
The following substances used in the Intralipid studies were infused through the jejunal catheter at a rate of 0.01 ml min−1 from t = 150 to 200 min: saline; 20% Intralipid (Baxter Healthcare Corporation, 0.02 kcal min−1); triacsin C (0.48 pmol min−1); Intralipid plus triacsin C (0.48 pmol min−1); tetracaine (0.01 mg min−1); or Intralipid plus tetracaine (0.01 mg min−1). MK-801, given at a rate of 0.03 ng min−1, was infused directly into the nucleus of the solitary tract for both glucose and lipid studies from 90 min to 200 min, similar to previous studies17,18.
Pancreatic-basal insulin clamp.
This procedure was performed as described in our previous studies17,18. The in vivo infusion experiments lasted a total of 200 min, with pancreatic clamps (insulin at 1.2 mU kg−1 min−1 and somatostatin at 3 μg kg−1 min−1) initiated at 90 min and gut infusions at 150 min. At the end of the experiment, rats were anesthetized and tissue samples were freeze-clamped in situ with steel tongs precooled in liquid nitrogen and stored at −80 °C.
Basal [3-3H]glucose infusion protocol (nonclamp conditions).
In separate groups of STZ-injected rats at 2 d after jejunal and vascular surgery, a primed-continuous infusion of [3-3H]glucose was initiated at 0 min and maintained throughout the entire experiment (total of 140 min). Jejunal infusions were initiated at 90 min and continued for the remaining 50 min. Of note, the rate of appearance of glucose (glucose production) is equal to the rate of disappearance of glucose (glucose uptake) in nonclamp conditions. At the end of the experiment, rats were anesthetized and tissue samples were freeze-clamped in situ with steel tongs precooled in liquid nitrogen and stored at −80 °C.
Fasting-refeeding protocol.
Rats were fasted beginning at 5 p.m. the day before the onset of the experiment (3 p.m.). Vehicle, phlorizin, triacsin C, or phlorizin plus triacsin C, at the same dose used during the clamp studies, was preinfused into the jejunum for 10 min, and then regular chow was given back to the rats ad libitum. The jejunal infusion was maintained for 50 min to match the treatment during the clamp studies. Food intake and glucose concentrations were measured every 10 min.
Biochemical analysis.
Plasma glucose concentrations were measured by the glucose oxidase method (Analox Instruments). For insulin and glucagon assays for clamp studies, blood was collected into heparinized tubes on ice. For insulin, glucagon and active GLP-1 assays in STZ-treated and BB-dp rats, blood was collected into tubes, on ice, containing aprotinin, EDTA and dipeptidyl peptidase IV (DPP-IV) inhibitor (Millipore). Plasma insulin and glucagon levels were determined by radioimmunoassay (Linco Research). Active GLP-1 plasma levels were determined by an ELISA (Linco Research).
Calculations.
Statistical analysis was performed by unpaired Student's t test and ANOVA as appropriate. Data are presented as means ± s.e.m. The time period 60–90 min was averaged for the basal condition, and the time period 180–200 min was averaged for the clamp condition.
Additional methods.
Detailed methodology is described in the Supplementary Methods.
References
Rubino, F., Schauer, P.R., Kaplan, L.M. & Cummings, D.E. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med. 61, 393–411 (2010).
Rubino, F. & Marescaux, J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann. Surg. 239, 1–11 (2004).
Wang, T.T. et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann. Surg. 247, 968–975 (2008).
Pacheco, D. et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am. J. Surg. 194, 221–224 (2007).
Kindel, T.L., Yoder, S.M., Seeley, R.J., D'Alessio, D.A. & Tso, P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J. Gastrointest. Surg. 13, 1762–1772 (2009).
Drewe, J., Gadient, A., Rovati, L.C. & Beglinger, C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology 102, 1654–1659 (1992).
Ogawa, N. et al. The vagal afferent pathway does not play a major role in the induction of satiety by intestinal fatty acid in rats. Neurosci. Lett. 433, 38–42 (2008).
Cohen, R.V. et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg. Obes. Relat. Dis. 3, 195–197 (2007).
Cohen, R.V. et al. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg. Obes. Relat. Dis. published online, doi:10.1016/j.soard.2012.01.017 (2 February 2012).
Badman, M.K. & Flier, J.S. The gut and energy balance: visceral allies in the obesity wars. Science 307, 1909–1914 (2005).
Cummings, D.E. & Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Invest. 117, 13–23 (2007).
Murphy, K.G. & Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859 (2006).
Coll, A.P., Farooqi, I.S. & O'Rahilly, S. The hormonal control of food intake. Cell 129, 251–262 (2007).
Lam, T.K. Neuronal regulation of homeostasis by nutrient sensing. Nat. Med. 16, 392–395 (2010).
Greenberg, D., Smith, G.P. & Gibbs, J. Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am. J. Physiol. 259, R110–R118 (1990).
Matzinger, D. et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 46, 688–693 (2000).
Wang, P.Y. et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452, 1012–1016 (2008).
Cheung, G.W., Kokorovic, A., Lam, C.K., Chari, M. & Lam, T.K. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 10, 99–109 (2009).
Lal, S., Kirkup, A.J., Brunsden, A.M., Thompson, D.G. & Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915 (2001).
Randich, A. et al. Jejunal administration of linoleic acid increases activity of neurons in the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R166–R173 (2004).
Ehrenkranz, J.R., Lewis, N.G., Kahn, C.R. & Roth, J. Phlorizin: a review. Diabetes Metab. Res. Rev. 21, 31–38 (2005).
Mordes, J.P., Bortell, R., Blankenhorn, E.P., Rossini, A.A. & Greiner, D.L. Rat models of type 1 diabetes: genetics, environment and autoimmunity. ILAR J. 45, 278–291 (2004).
Cohen, R., Pinheiro, J.S., Correa, J.L. & Schiavon, C.A. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg. Obes. Relat Dis. 2, 401–404 (2006).
Kim, Z. & Hur, K.Y. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J. Surg. 35, 631–636 (2011).
Thaler, J.P. & Cummings, D.E. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150, 2518–2525 (2009).
Rubino, F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31 (suppl. 2), S290–S296 (2008).
Cox, J.E., Kelm, G.R., Meller, S.T., Spraggins, D.S. & Randich, A. Truncal and hepatic vagotomy reduce suppression of feeding by jejunal lipid infusions. Physiol. Behav. 81, 29–36 (2004).
Troy, S. et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 8, 201–211 (2008).
Chambers, A.P. et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141, 950–958 (2011).
Patti, M.E. et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 17, 1671–1677 (2009).
Watkins, J.B. III & Dykstra, T.P. Alterations in biliary excretory function by streptozotocin-induced diabetes. Drug Metab. Dispos. 15, 177–183 (1987).
Breen, D.M. et al. Duodenal PKC-δ and cholecystokinin signaling axis regulates glucose production. Diabetes 60, 3148–3153 (2011).
Kokorovic, A. et al. Duodenal mucosal protein kinase C-δ regulates glucose production in rats. Gastroenterology 141, 1720–1727 (2011).
Acknowledgements
We are extremely grateful to P.Y.T. Wang for excellent technical assistance. This work was supported by a research grant to T.K.T.L. from the Canadian Institutes of Health Research (MOP-82701). D.M.B. is supported by a post-doctoral fellowship from the University Health Network and the Banting and Best Diabetes Centre (BBDC), University of Toronto. B.A.R. is supported by a BBDC graduate scholarship. A.K. and G.W.C.C. were supported by Canadian Institutes of Health Research and BBDC graduate scholarships. T.K.T.L. holds the John Kitson McIvor (1915–1942) Endowed Chair in Diabetes Research and the Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
Author information
Authors and Affiliations
Contributions
D.M.B. conducted and designed experiments, performed data analyses and wrote the manuscript; B.A.R., A.K. and G.W.C.C. assisted in experiments; R.W. assisted in setting up the DJB surgical procedure; and T.K.T.L. supervised the project, designed experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3 and Supplementary Methods (PDF 2324 kb)
Rights and permissions
About this article
Cite this article
Breen, D., Rasmussen, B., Kokorovic, A. et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 18, 950–955 (2012). https://doi.org/10.1038/nm.2745
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2745
This article is cited by
-
The Significance of Bile in the Biliopancreatic Limb on Metabolic Improvement After Duodenal-Jejunal Bypass
Obesity Surgery (2024)
-
Effect of duodenal-jejunal bypass on diabetes in the early postoperative period
Scientific Reports (2023)
-
Current status of metabolic surgery in patients with type I diabetes mellitus and obesity: a nationwide multicenter study
Langenbeck's Archives of Surgery (2023)
-
Temporal Relationship Between Insulin Resistance and Lipid Accumulation After Bariatric Surgery: a Multicenter Cohort Study
Obesity Surgery (2023)
-
Choice of Bariatric Surgery in Patients with Obesity and Type 1 Diabetes Mellitus? an Up-to-Date Systematic Review
Obesity Surgery (2022)