Abstract
Calorie restriction (CR) is defined as a reduction in calorie intake below the usual ad libitum intake without malnutrition. Ample of clinical and experimental evidence has demonstrated that CR is capable of retarding aging process and development of cardiovascular disease. Although suppression of reactive oxygen species production and inflammation plays a central role in the favorable cardiovascular effects of CR, the health benefit of CR is believed to be ultimately mediated through a cadre of biochemical and cellular adaptations including redox homeostasis, mitochondrial function, inflammation, apoptosis and autophagy. Despite the apparent beneficial cardiovascular effects of CR, implementation of CR in the health care management is still hampered by apparent applicability issues and health concerns. Here we briefly review the cardiac consequence of CR and discuss whether CR may represent a safe and effective strategy in the management of cardiovascular health.
Similar content being viewed by others
Calorie restriction and health
Calorie restriction (CR) is defined as a reduction in calorie intake below the usual ad libitum intake without malnutrition. In general, the daily caloric intake subjected to CR has been restricted to 50% to 70% of the average food intake in subjects eating ad libitum1, 2. CR was first reported to retard aging and prolong median and maximal life span in the 1930s3. Over the last several decades, CR has been widely studied which exhibits an apparent beneficial impact on longevity, age-associated diseases, and attenuation of functional decline, across a broad variety of species (including rats, mice, fish, flies, worms and yeast)4, 5. Moreover, CR provides protection against a cadre of chronic diseases including diabetes mellitus, neurodegenerative diseases, autoimmune disorders6, 7, 8 and cancer9, 10, 11, 12. CR has been shown to alter a variety of physiological parameters especially reduced metabolic rate and oxidative damage, resulting in a decrease in the incidence of cardiovascular diseases13. In response to energy deficiency, experimental animals experience a drastic decrease in both fat mass and lean body mass. Muscle mass is overtly reduced, although the rate of loss of function and mass/body weight with the aging process is attenuated9, 14, 15, 16. Metabolic rate may decrease transiently although studies have indicated similar metabolic rate per kg lean body mass to that of ad libitum fed rodents in long-term CR protocol17. Body temperature, systolic and diastolic blood pressure all decrease18, 19 along with the reduced sympathetic activity20. CR-treated animals are more spontaneously active and display superior cognitive abilities compared to their ad libitum counterparts21, 22.
The most prominent mechanism that may be responsible for the beneficial health and physiological effects of CR are usually mediated through increased insulin sensitivity which results in reduced plasma glucose and insulin concentrations and improved glucose tolerance23; reduced levels of oxidative stress (decreased oxidative damage to proteins, lipids and DNA)24, increased resistance to various types of stress including heat, oxidative and metabolic stress25 as well as enhanced immune function26.
Impact of CR on aging
An inverse relationship between calorie intake and lifespan has been revealed in mice, suggesting a key role for regulators of energy metabolism in the mechanism of CR. Accordingly, CR-induced metabolic reprogramming may be a key event in the mechanism of lifespan extension27, 28. The age when CR is started, the severity of restriction, and strain or genetic background of animals determine the magnitude of life extension. The only mammals in which CR has clearly been shown to slow primary aging and extend maximum lifespan are rats and mice29, 30, 31. In rodents, initiating a 30% to 60% reduction in calorie intake below usual ad libitum intake early in life (from shortly after weaning to age 6 months) caused a proportionate 30% to 60% increase in maximum lifespan, whereas a 44% reduction in calorie intake started in adulthood (12 months) extended maximum lifespan by only 10% to 20%32. Data from rodents found that CR increases longevity by preventing or delaying chronic diseases including diabetes, atherosclerosis, cardiomyopathy, autoimmune diseases, kidney and respiratory diseases, and cancer3, 30, 31, 32. In addition, CR is capable of decreasing neurodegeneration in the brain and enhancing neurogenesis in animal models of Alzheimer's disease, Parkinson disease, Huntington disease and stroke27, 32, 33, 34. However, reduction of chronic diseases does not completely explain the increased lifespan and preservation of function at more youthful-like states in calorie-restricted rodents. In particular, approximately one third of these experimental rodents die without any evidence of apparent organ pathology35.
More recent studies suggest that reducing calorie intake can also increase the lifespan in nonhuman primates36, 37. There are two longevity studies (one at the University of Wisconsin, the other at the National Institute on Aging) examining the long-term effects of CR on aging in rhesus monkeys38. Up-to-now, the experimental data have shown that a number of metabolic, hormonal and structural adaptations taken place in CR-treated rodents also exist in CR monkeys. A 20-year longitudinal adult-onset CR study in rhesus monkeys at the Wisconsin National Primate Research Center revealed that moderate CR lowered the incidence of aging-related death. Fifty percent of control fed animals survived as compared with 80% of the CR animals. Furthermore, CR delayed the onset of age-associated pathologies. Specifically, CR reduced the incidence of diabetes, cancer, cardiovascular disease, and brain atrophy27. In addition, immune senescence and sarcopenia are attenuated in calorie restricted monkeys39. Taken together, emerging evidence from ongoing research of CR in monkeys suggest that this nutritional paradigm may be universal among species with regards to extension of life span and retardation of aging.
It is likely that certain physiological and psychological consequences elicited by CR seen in animals may distinctly impact human life. It is rather difficult to determine whether CR has beneficial effects on longevity in humans because there are little validated biomarkers that may serve as surrogate markers of aging. Furthermore, it is impractical to conduct randomized, diet-controlled, long-term survival studies in human40. Nonetheless, data from epidemiological studies suggest that CR may offer beneficial effects on the factors involved in the pathogenesis of primary and secondary aging and life expectancy in humans. Data from a series of studies conducted by the Calorie Restriction Society, a group that practices self-imposed CR with a belief that diet restriction extends lifespan, were recently reported41, 42, 43. Compared with control individuals consuming a Western diet, calorie restricted individuals exhibited similar alterations in metabolic and organ function reported previously in calorie restricted rodents. The main parameters for metabolic and organ function include low percentage of body fat, low systolic and diastolic blood pressure, markedly improved lipid profile, increased insulin sensitivity, reduced plasma concentration of inflammatory markers, low circulating growth factors, and low serum concentration of T341, 42, 43. Interestingly, left ventricular diastolic function (ie, parameters of viscoelasticity and stiffness) in calorie restricted individuals was somewhat similar to those who were ∼16 years younger43 and is consistent with the beneficial cardiac effects of CR seen in mice44. Nonetheless, further large scale study is in need to determine the human metabolic and functional adaptive responses to CR.
Impact on cardiovascular system
CR exerts a protective effect on cardiovascular system. Evidence from both experimental animals and human has demonstrated that CR decreases basal heart rate29, 45. In addition, Mager and coworkers recently depicted that rats maintained on a CR diet display increased heart rate variability46. High heart rate variability is usually associated with improved cardiovascular function, whereas low heart rate variability is usually indicative of poor cardiovascular function. Other than heart rate, CR may also participate in the regulation of blood pressure. Hypertension is a major risk factor for coronary artery disease and stroke47. Both systolic and diastolic blood pressures are significantly reduced in rats maintained on a CR diet48. Similarly, monkeys also display reduced blood pressure following CR diet37.
Progressive CR induces a dose-dependent increase in myocardial triglyceride content and a dose-dependent decrease in diastolic function in lean healthy men49. Viljanen and colleagues showed that myocardial free fatty acid uptake was reduced after a short-term low calorie diet resulting in overt weight loss. Furthermore, these changes were in parallel with the reduction of left ventricular mass, cardiac work, and perfusion at rest and a subtle reduction in myocardial triglyceride content50.
It has been shown that diastolic dysfunction is favorably affected by CR in human43. Interestingly, changes of diastolic function in CR subjects were very similar to lifelong CR mice44. CR possesses cardiac-specific effects which may offset aging-associated changes in diastolic function. These beneficial effects on cardiac function might be mediated by the effect of CR on blood pressure, systemic inflammation and myocardial fibrosis43. Further evidence revealed notable effect of CR on endothelial function22. CR is capable of improving endothelium-dependent vasodilatation51. It is plausible to speculate that CR improves endothelial function in the nonobese, probably via decreased production of ROS. CR lowers most major coronary heart disease (CHD) risk factors, including plasma low density lipoprotein cholesterol (LDL-C) concentration, total cholesterol/high density lipoprotein cholesterol (HDL-C) ratio, C-reactive protein (CRP) concentrations, and the homeostasis model assessment for insulin resistance (HOMA-IR) index52.
CR reduces levels of oxidative stress in cardiovascular system by alleviating oxidative modifications of proteins and DNA and decreased levels of lipid peroxidation in the heart24, 53, 54. CR reduces inflammatory processes which triggers atherosclerosis, as indicated by reduced levels of leukocytes and circulating levels of tumor necrosis factor α (TNF-α) and other inflammatory cytokines1, 55. By suppressing atherosclerosis, CR should ultimately reduce the risk of cardiovascular disease and stroke.
Effect of CR on cardiovascular disease and cardiopathology
Much of the cardiovascular disease is related to the metabolic syndrome which diagnosis standard including clinical findings of increased abdominal circumference, elevated triglycerides, low high-density lipoprotein-cholesterol, elevated fasting blood glucose and/or elevated blood pressure. It is well established that, beside drug therapy, lifestyle therapies that combine energy restriction and physical activity independently improve a number of cardiovascular disease risk factors including insulin resistance, impaired glucose tolerance, dyslipidemia and hypertension56, 57, 58.
Hypertension and cardiac hypertrophy
Reduced caloric intake lowers blood pressure in hypertension59, 60, 61 and obesity62, which are usually accompanied with overt cardiovascular anomalies. The mechanisms responsible for this observation have not been clarified. However, decreases in sympathetic nervous activity frequently accompany reduced caloric intake. In the spontaneously hypertensive rats, fasting-induced reduction in blood pressure is accompanied by reduced cardiac norepinephrine turnover63 and reduced sympathetic support of blood pressure60. Reduced plasma norepinephrine levels and decreased sympathetic support of blood pressure have also been observed in aortic coarctation-induced hypertension following CR59. There is thus considerable evidence suggesting that reduced sympathetic activity may serve as an important mechanism behind the reduction in blood pressure following decreased caloric intake64.
The Dahl salt-sensitive rat is model which indices of decompensated, pressure-overload hypertrophy. Seymour reported that CR reduced the degree of change rather than preventing it. CR reduced restrictive pattern development (lower E/A), prolonged early filling deceleration time, and shortened relaxation time. These authors concluded that modest CR, independent of salt intake, reduced hypertension associated decompensated pressure-overload hypertrophy65.
Ischemia and reperfusion
Shinmura showed that short-term (2 weeks) CR is capable of improving myocardial ischemic tolerance in both young and old Fischer 344 rats. The cardioprotection induced by CR is associated with an increase in AMPK activation66. Furthermore prolonged CR (6 months) improves myocardial ischemic tolerance and restores ischemic preconditioning (IP) effect in middle-aged rats, possible through a nitric oxide-dependent increase in nuclear human silent information regulator type 1 (SIRT1) content67. CR also promoted ischemia-induced revascularization in wild-type mice but not adiponectin knockout mice. Adiponectin is known to promote vascular cell function and survival under stressed conditions66, 67. It was recently reported that CR may confer resistance to myocardial ischemia-reperfusion injury by increasing adiponectin levels68. Lifelong CR drastically attenuates myocardial oxidative stress during ischemia/reperfusion23 and post-ischemic inflammatory response69.
IP is able to protect the heart against ischemia reperfusion damage in adult but not in senescent rat hearts. Abete and colleagues found that IP reduces postischemic dysfunction in the hearts from adult and food-restricted but not in the ad libitum-fed senescent rats70. Nonetheless, exercise training and food restriction individually produce partial preservation of IP in the aging heart71. One of the mechanisms responsible for early IP conservation in aging heart may be restoration of the norepinephrine release in response to preconditioning stimulus.
Diabetes mellitus and metabolic syndrome
Major metabolic effects of substantial weight loss in obese patients with type 2 diabetes provide an avenue to understand the mechanisms behind metabolic syndrome. Typical diagnosis of metabolic syndrome requires presence of 3 of 5 characteristics: increased abdominal waist, hyperglycemia, high blood triglycerides, high blood pressure and HDL-C. One of the commonly accepted hypotheses is that the crucial initial event is the increased concentration of circulating free fatty acids (FFAs) and cytokines derived from excess visceral abdominal fat72. Increased circulating FFAs is known to decrease glucose uptake by heart and skeletal muscle72 although it is rather difficult to link chronically increased circulating FFAs to increased blood triglycerides and decreased high density lipoprotein (HDL) in humans. Weight loss in obesity led to decreased waist circumference, decreased circulating glucose and triglycerides, and cytokines. Conversely, increased circulating FFAs taken up by the heart promote accumulation of myocardial triglycerides leading to diastolic dysfunction and lipotoxic cardiomyopathy in human73. Overall, these results provide support the concept that excess circulating FFA, as associated with abdominal visceral obesity, is fundamental in the pathogenesis of an increasingly common human disease, namely, metabolic syndrome. It was also indicated that unloading the human body of adipose tissue induces a “reverse metabolic syndrome.” Similar to human, type 2 diabetic rats undergo CR or exercise displayed improved plasma levels of glucose, insulin, cholesterol and triacylglycerol and reduced abdominal fat accumulation74. Other report also reported reduction in cardiovascular disease risk in type 2 diabetes following CR75. Hammer and colleagues reported that a short-term very low-calorie diet increases myocardial triglyceride content and is associated with a decrease in left ventricular diastolic function in patients with well-controlled type 2 diabetes76. Furthermore prolonged caloric restriction improves glucoregulation associated with decreased myocardial triglyceride content and favorable effects on blood pressure and myocardial function in insulin-treated obese patients with type 2 diabetes77. These data prove that myocardial triglyceride stores in obese patients with type 2 diabetes are flexible and amendable to therapeutic intervention by caloric restriction.
Other heart diseases
CR reduces the severity of spontaneous cardiomyopathy in rats and prevents age-associated alterations in late diastolic function in mice78. CR also improves the survival and myocardial damage in obese mice with viral myocarditis, which is accompanied by increased adiponectin levels in plasma and myocardium79. In addition, CR attenuates atherosclerotic formation34.
Signal transduction mechanism involved in CR
Evidence from animal models and preliminary studies in humans indicates that CR delays cardiac aging and prevents cardiovascular disease. These effects are mediated by a wide spectrum of biochemical and cellular adaptations, including redox homeostasis, mitochondrial function80, inflammation81, 82, apoptosis83, and autophagy84. Oxidative stress plays an important role in the pathogenesis of coronary artery disease by mediating expression of inflammatory genes and eliciting oxidative modification of lipoprotein particles85, 86. CR seems to confer vasoprotection through attenuation of oxidative stress and antiinflammatory effects in aged animals13. CR also increases bioavailability of antiatherogenic NO and improves endothelial function13. In addition, CR exerts beneficial effects on a range of systemic cardiovascular risk factors87, 88.
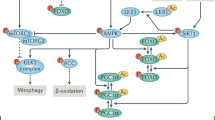
Over the last decades, a number of nutrient-sensitive proteins have been identified in the health and longevity effects of CR, including the sirtuins, forkhead box transcription factors (FOXOs)89 and mammalian target of rapamycin (mTOR)90. Corton and Brown-Borg provided compelling evidence for the broad participation of the nuclear receptor transcription factor, peoxisome proliferator activated receptor (PPAR) α in CR91. The PPAR γ co-activator PGC-1α is a key regulator of genes involved in mitochondrial metabolism. CR increases mRNA levels of PGC-1α in multiple tissues92. Regulation of mitochondria through manipulation of SIRT1 and glycogen synthase kinase 3 beta (GSK3β) is a common feature of CR and the stress response93. Other transcription factors have also been considered with a key role in CR-elicited biological actions. Heydari and colleagues reported that CR enhanced the heat shock transcription factor 1 (HSF-1) function and thereby promoted the transcription of the important chaperone, heat shock protein 70 (HSP70)94. Kim and coworkers proposed an important role of the following redox-sensitive transcription factors in CR-associated actions including nuclear factor kappa B (NF-κB), activator protein-1 (AP-1); and hypoxia inducible factor-1 (HIF-1)95. However, much still remains to be determined with regards to the functioning of transcription factors in long-term CR. Table 1 summaries some of the most important signaling molecules involved in CR-induced biological and physiological responses.
Conclusion and perspectives
When considering all possible aging interventions evaluated, there is little doubt that CR remains the most robust. Studies in numerous species have demonstrated that CR can increase lifespan, reduce the incidence and delay the onset of age-related diseases, improve stress resistance, decelerate functional decline, and in particular, exert cardioprotection. One of the most pertinent issues in CR research is the relevance of this nutritional intervention in human aging, given the practicality of long term CR in human. More recent research has targeted on the development of “CR mimetics”, namely compounds mimicking favorable metabolic effect of CR without restricting caloric intake. Certain compounds, such as resveratrol and rapamycin, have shown some clinical promises with many CR-like effects to promote cardiovascular health and retard cardiac aging in humans96.
References
Spaulding CC, Walford RL, Effros RB . Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev 1997; 93: 87–94.
Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT . Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol 1982; 37: 130–41.
McCay CM, Crowell MF, Maynard LA . The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 1989; 5: 155–71; discussion 172.
Nutrition classics. The American Journal of Cancer, Volume XXXVIII, March, 1940: The initiation and growth of tumors. Introduction. I. Effects of underfeeding. By Albert Tannenbaum. Nutr Rev 1987; 45: 20–2.
Kritchevsky D . Caloric restriction and experimental carcinogenesis. Hybrid Hybridomics 2002; 21: 147–51.
Weindruch R, Walford RL, Fligiel S, Guthrie D . The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 1986; 116: 641–54.
Roe FJ, Lee PN, Conybeare G, Kelly D, Matter B, Prentice D, et al. The Biosure Study: influence of composition of diet and food consumption on longevity, degenerative diseases and neoplasia in Wistar rats studied for up to 30 months post weaning. Food Chem Toxicol 1995; 33: 1S–100S.
Bronson RT, Lipman RD . Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging 1991; 55: 169–84.
Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL . The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol 1983; 38: 420–30.
Tannenbaum A, Silverstone H . The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res 1949; 9: 724–7.
Thompson HJ, Zhu Z, Jiang W . Dietary energy restriction in breast cancer prevention. J Mammary Gland Biol Neoplasia 2003; 8: 133–42.
Pugh TD, Oberley TD, Weindruch R . Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res 1999; 59: 1642–8.
Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R . Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 2008; 102: 519–28.
Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM . Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J 1997; 11: 573–81.
Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, et al. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol 2003; 284: R474–80.
Payne AM, Dodd SL, Leeuwenburgh C . Life-long calorie restriction in Fischer 344 rats attenuates age-related loss in skeletal muscle-specific force and reduces extracellular space. J Appl Physiol 2003; 95: 2554–62.
Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR . Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev 2005; 126: 783–93.
Hunt LM, Hogeland EW, Henry MK, Swoap SJ . Hypotension and bradycardia during caloric restriction in mice are independent of salt balance and do not require ANP receptor. Am J Physiol Heart Circ Physiol 2004; 287: H1446–51.
Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW . Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev 1989; 48: 117–33.
Kushiro T, Kobayashi F, Osada H, Tomiyama H, Satoh K, Otsuka Y, et al. Role of sympathetic activity in blood pressure reduction with low calorie regimen. Hypertension 1991; 17: 965–8.
Means LW, Higgins JL, Fernandez TJ . Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav 1993; 54: 503–8.
Bellush LL, Wright AM, Walker JP, Kopchick J, Colvin RA . Caloric restriction and spatial learning in old mice. Physiol Behav 1996; 60: 541–7.
Heilbronn LK, Ravussin E . Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 2003; 78: 361–9.
Sohal RS, Weindruch R . Oxidative stress, caloric restriction, and aging. Science 1996; 273: 59–63.
Mattson MP, Chan SL, Duan W . Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev 2002; 82: 637–72.
Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN . Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci 2009; 64: 1107–13.
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009; 325: 201–4.
Cox LS, Mattison JA . Increasing longevity through caloric restriction or rapamycin feeding in mammals: common mechanisms for common outcomes? Aging Cell 2009; 8: 607–13.
Weindruch R . The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol 1996; 24: 742–5.
Weindruch R . Caloric restriction and aging. Sci Am 1996; 274: 46–52.
Masoro EJ . Overview of caloric restriction and ageing. Mech Ageing Dev 2005; 126: 913–22.
Mattson MP . Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr 2005; 25: 237–60.
Martin B, Mattson MP, Maudsley S . Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 2006; 5: 332–53.
Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, et al. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev 2002; 123: 1121–31.
Shimokawa I, Higami Y, Hubbard GB, McMahan CA, Masoro EJ, Yu BP . Diet and the suitability of the male Fischer 344 rat as a model for aging research. J Gerontol 1993; 48: B27–32.
Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC . Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci 2003; 58: 212–9.
Mattison JA, Lane MA, Roth GS, Ingram DK . Calorie restriction in rhesus monkeys. Exp Gerontol 2003; 38: 35–46.
Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB . Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol 1993; 48: B17–26.
Roth GS, Ingram DK, Lane MA . Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc 1999; 47: 896–903.
Johnson TE . Recent results: biomarkers of aging. Exp Gerontol 2006; 41: 1243–6.
Fontana L, Meyer TE, Klein S, Holloszy JO . Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004; 101: 6659–63.
Fontana L, Klein S, Holloszy JO, Premachandra BN . Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab 2006; 91: 3232–5.
Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L . Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 2006; 47: 398–402.
Taffet GE, Pham TT, Hartley CJ . The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci 1997; 52: B285–90.
Thomas J, Bertrand H, Stacy C, Herlihy JT . Long-term caloric restriction improves baroreflex sensitivity in aging Fischer 344 rats. J Gerontol 1993; 48: B151–5.
Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J 2006; 20: 631–7.
Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M . Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 2006; 7: 173–7.
Young JB, Mullen D, Landsberg L . Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism 1978; 27: 1711–4.
Hammer S, van der Meer RW, Lamb HJ, Schar M, de Roos A, Smit JW, et al. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab 2008; 93: 497–503.
Viljanen AP, Karmi A, Borra R, Parkka JP, Lepomaki V, Parkkola R, et al. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol 2009; 103: 1721–6.
Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Sasaki S, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens 2002; 15: 302–9.
Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab 2007; 293: E197–202.
Faine LA, Diniz YS, Almeida JA, Novelli EL, Ribas BO . Toxicity of ad lib. overfeeding: effects on cardiac tissue. Food Chem Toxicol 2002; 40: 663–8.
Pamplona R, Portero-Otin M, Requena J, Gredilla R, Barja G . Oxidative, glycoxidative and lipoxidative damage to rat heart mitochondrial proteins is lower after 4 months of caloric restriction than in age-matched controls. Mech Ageing Dev 2002; 123: 1437–46.
Muthukumar A, Zaman K, Lawrence R, Barnes JL, Fernandes G . Food restriction and fish oil suppress atherogenic risk factors in lupus-prone (NZB x NZW) F1 mice. J Clin Immunol 2003; 23: 23–33.
Maggio CA, Pi-Sunyer FX . Obesity and type 2 diabetes. Endocrinol Metab Clin North Am 2003; 32: 805–22, viii.
Wagh A, Stone NJ . Treatment of metabolic syndrome. Expert Rev Cardiovasc Ther 2004; 2: 213–28.
Hamdy O, Goodyear LJ, Horton ES . Diet and exercise in type 2 diabetes mellitus. Endocrinol Metab Clin North Am 2001; 30: 883–907.
VanNess JM, Casto RM, DeMaria JE, Overton JM . Food restriction attenuates the blood pressure response to paraventricular hypothalamic nuclei lesions in aortic coarctation hypertension. Brain Res 1998; 792: 237–45.
Overton JM, VanNess JM, Casto RM . Food restriction reduces sympathetic support of blood pressure in spontaneously hypertensive rats. J Nutr 1997; 127: 655–60.
Ernsberger P, Nelson DO . Effects of fasting and refeeding on blood pressure are determined by nutritional state, not by body weight change. Am J Hypertens 1988; 1: 153S–57S.
Apfelbaum M . Adaptation to changes in caloric intake. Prog Food Nutr Sci 1978; 2: 543–59.
Young JB, Landsberg L . Suppression of sympathetic nervous system during fasting. Science 1977; 196: 1473–5.
VanNess JM, DeMaria JE, Overton JM . Increased NPY activity in the PVN contributes to food-restriction induced reductions in blood pressure in aortic coarctation hypertensive rats. Brain Res 1999; 821: 263–9.
Seymour EM, Parikh RV, Singer AA, Bolling SF . Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol 2006; 41: 661–8.
Shinmura K, Tamaki K, Bolli R . Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol 2005; 39: 285–96.
Shinmura K, Tamaki K, Bolli R . Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 2008; 295: H2348–55.
Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R . Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 2007; 116: 2809–17.
Chandrasekar B, Nelson JF, Colston JT, Freeman GL . Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2001; 280: H2094–102.
Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, et al. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol 2002; 282: H1978–87.
Abete P, Testa G, Galizia G, Mazzella F, Della Morte D, de Santis D, et al. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol 2005; 40: 43–50.
Opie LH . Metabolic syndrome. Circulation 2007; 115: e32–5.
Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005; 54: 3148–53.
Sakamoto S, Minami K, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, et al. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous NIDDM. Diabetes 1998; 47: 82–6.
Harder H, Dinesen B, Astrup A . The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients. Int J Obes Relat Metab Disord 2004; 28: 180–2.
Hammer S, van der Meer RW, Lamb HJ, de Boer HH, Bax JJ, de Roos A, et al. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2008; 295: E714–8.
Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008; 52: 1006–12.
Kemi M, Keenan KP, McCoy C, Hoe CM, Soper KA, Ballam GC, et al. The relative protective effects of moderate dietary restriction versus dietary modification on spontaneous cardiomyopathy in male Sprague-Dawley rats. Toxicol Pathol 2000; 28: 285–96.
Kanda T, Saegusa S, Takahashi T, Sumino H, Morimoto S, Nakahashi T, et al. Reduced-energy diet improves survival of obese KKAy mice with viral myocarditis: induction of cardiac adiponectin expression. Int J Cardiol 2007; 119: 310–8.
Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L . Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res 2010; 13: 65–74.
Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res 2009; 58: 143–50.
Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 2009; 130: 518–27.
Deng X, Cheng J, Zhang Y, Li N, Chen L . Effects of caloric restriction on SIRT1 expression and apoptosis of islet beta cells in type 2 diabetic rats. Acta Diabetol 2009. doi: 10.1007/s00592-009-0159-7
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C . Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 2010; 45: 138–48.
Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 2002; 90: 1159–66.
Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G . Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 2003; 17: 1183–5.
Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP . Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults — a randomized clinical trial. J Gerontol A Biol Sci Med Sci 2009; 64: 90–5.
Hecker KD, Kris-Etherton PM, Zhao G, Coval S, St Jeor S . Impact of body weight and weight loss on cardiovascular risk factors. Curr Atheroscler Rep 1999; 1: 236–42.
Greer EL, Brunet A . FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005; 24: 7410–25.
Schieke SM, Finkel T . Mitochondrial signaling, TOR, and life span. Biol Chem 2006; 387: 1357–61.
Corton JC, Brown-Borg HM . Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci 2005; 60: 1494–509.
Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005; 310: 314–7.
Anderson RM, Barger JL, Edwards MG, Braun KH, O'Connor CE, Prolla TA, et al. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 2008; 7: 101–11.
Heydari AR, You S, Takahashi R, Gutsmann A, Sarge KD, Richardson A . Effect of caloric restriction on the expression of heat shock protein 70 and the activation of heat shock transcription factor 1. Dev Genet 1996; 18: 114–24.
Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY . Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev 2002; 123: 1589–95.
Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C . Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med 2009; 25: 715–32, ix.
Kim DH, Kim JY, Yu BP, Chung HY . The activation of NF-kappa B through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology 2008; 9: 33–47.
Ciaraldi TP, Oh DK, Christiansen L, Nikoulina SE, Kong AP, Baxi S, et al. Tissue-specific expression and regulation of GSK-3 in human skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 2006; 291: E891–8.
Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, et al. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology 2005; 6: 27–37.
Eitam H, Brosh A, Orlov A, Izhaki I, Shabtay A . Caloric stress alters fat characteristics and Hsp70 expression in milk somatic cells of lactating beef cows. Cell Stress Chaperones 2009; 14: 173–82.
Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila Pa) 2008; 1: 65–76.
Masternak MM, Bartke A . PPARs in Calorie Restricted and Genetically Long-Lived Mice. PPAR Res 2007; 2007: 28436.
Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 2008; 22: 1753–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Ren, J. Caloric restriction and heart function: is there a sensible link?. Acta Pharmacol Sin 31, 1111–1117 (2010). https://doi.org/10.1038/aps.2010.146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.146
Keywords
This article is cited by
-
Molecular and cellular pathways contributing to brain aging
Behavioral and Brain Functions (2021)
-
Caloric restriction reduces sympathetic activity similar to beta-blockers but conveys additional mitochondrio-protective effects in aged myocardium
Scientific Reports (2021)
-
Health Benefits of Fasting and Caloric Restriction
Current Diabetes Reports (2017)
-
Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats
Acta Pharmacologica Sinica (2014)
-
Age-related cardiovascular disease and the beneficial effects of calorie restriction
Heart Failure Reviews (2012)