Abstract
Background and Objective
Dulaglutide is a long-acting glucagon-like peptide-1 receptor agonist administered as once-weekly subcutaneous injections for the treatment of type 2 diabetes (T2D). The clinical pharmacokinetics of dulaglutide were characterized in patients with T2D and healthy subjects.
Methods
The pharmacokinetics of dulaglutide were assessed throughout clinical development, including conventional pharmacokinetic analysis in clinical pharmacology studies and population pharmacokinetic analyses of data from combined phase 2 and phase 3 studies in patients with T2D. The effects of potential covariates on dulaglutide population pharmacokinetics were evaluated using nonlinear mixed-effects models.
Results
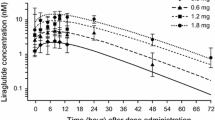
Dulaglutide gradually reached the maximum concentration in 48 h and had a terminal elimination half-life of 5 days. Steady state was achieved between the second and fourth doses. The accumulation ratio was 1.56 for the 1.5 mg dose. Intra-individual variability estimates for the area under the plasma concentration–time curve and the maximum concentration were both <17 % [coefficient of variation (CV)]. There was no difference in pharmacokinetics between injection sites (arm, thigh or abdomen). Dulaglutide pharmacokinetics were well described by a two-compartment model with first-order absorption and elimination. The population clearance was estimated at 0.126 L/h [inter-individual variability (CV) 33.8 %]. Age, body weight, sex, race and ethnicity did not influence dulaglutide pharmacokinetics to any clinically relevant degree.
Conclusion
The pharmacokinetics of dulaglutide support once-weekly administration in patients with T2D. The pharmacokinetic findings suggest that dose adjustment is not necessary on the basis of body weight, sex, age, race or ethnicity or site of injection.





Similar content being viewed by others
References
Trulicity (prescribing information). Indianapolis: Lilly USA, LLC; 2014. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125469Orig1s000Lbl.pdf Accessed 06 Feb 2015.
Sanford M. Dulaglutide: first global approval. Drugs. 2014;74(17):2097–103.
Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287–96.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68.
Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011;3(5):422–30.
Barrington P, Chien JY, Showalter HD, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:426–33.
Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab. 2011;3:434–8.
Thompson AM, Trujillo JM. Dulaglutide: the newest GLP-1 receptor agonist for the management of type 2 diabetes. Ann Pharmacother. 2015;49(3):351–9.
Skrivanek Z, Gaydos BL, Chien JY, Geiger MJ, Heathman MA, Berry S, Anderson JH, Forst T, Milicevic Z, Berry D. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5). Diabetes Obes Metab. 2014;16(8):748–56.
Martin S, Cui X, Heathman M, de la Peña A, Harper K. The nausea profile of once weekly dulaglutide 1.5 mg. In: Presented at the American association of pharmaceutical scientists annual meeting and exposition, San Diego; 2014.
Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin Ther. 2006;28(12):1989–95.
Watson E, Jonker DM, Jacobsen LV, Ingwersen SH. Population pharmacokinetics of liraglutide, a once-daily human glucagon-like peptide-1 analog, in healthy volunteers and subjects with type 2 diabetes, and comparison to twice-daily exenatide. J Clin Pharmacol. 2010;50(8):886–94.
Nauck M, Weinstock R, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58.
Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added on to pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67.
Umpierrez GE, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy vs metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168–76.
Acknowledgments
This work was sponsored by Eli Lilly and Company. The authors thank the trial investigators, trial staff and trial participants for their contributions. The authors also express their gratitude to Dr. Helen Salter and Ms. Lisa Toth for providing medical writing support, and to Dr. Jessie L. Fahrbach, Dr. Sherry Martin, Dr. Malcolm Mitchell, Dr. Archana Chaudhary, Dr. Karen Schneck, Dr. Lai San Tham and Mr. Siak Leng Choi for providing scientific review and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations of interest
JSG, MAH, XC, JM, CL, JYC and AdlP are employees and shareholders of Eli Lilly and Company.
Scientific meeting presentation
This work was presented in part at the American Diabetes Association’s 74th Scientific Sessions in San Francisco, CA, USA, in June 2014, and at the European Association for the Study of Diabetes 50th Annual Meeting in Vienna, Austria, in September 2014.
Ethical standards
All clinical studies described herein were approved by the appropriate ethics committee and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave their informed consent prior to their inclusion in the studies. This manuscript does not contain animal studies or data.
Rights and permissions
About this article
Cite this article
Geiser, J.S., Heathman, M.A., Cui, X. et al. Clinical Pharmacokinetics of Dulaglutide in Patients with Type 2 Diabetes: Analyses of Data from Clinical Trials. Clin Pharmacokinet 55, 625–634 (2016). https://doi.org/10.1007/s40262-015-0338-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0338-3