Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of drugs that have been extensively investigated for the treatment of hyperglycemia in type 2 diabetes mellitus (T2DM). These drugs reduce hyperglycemia by blocking renal glucose reabsorption, thereby promoting increased renal glucose excretion. Beyond glycemic control, these drugs have other beneficial effects on cardiovascular (CV) risk factors. The present review discusses the potential role of SGLT2 inhibitors in treating CV complications (acute and chronic) associated with T2DM.
Funding: AstraZeneca Pharma India Ltd.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) have reached epidemic proportions worldwide, becoming increasingly pressing public health problems. The prevalence of T2DM ranges from 6.9% to 10.2% in developed countries, and exceeds 7% in developing countries [1, 2]. CVD accounts for 17.5 million deaths annually, an estimated 31% of all deaths worldwide [3].
A growing body of evidence suggests strong links between T2DM, CVD and hypertension [high blood pressure (BP)]. Firstly, T2DM is known to be a major risk factor for cardiovascular (CV) morbidity and mortality [4]. In addition to microvascular complications (nephropathy, retinopathy and neuropathy), T2DM is associated with macrovascular complications including CVD, cerebrovascular disease and peripheral artery disease. Secondly, the majority of patients with T2DM are overweight or obese, a further risk factor for both CVD and hypertension [5].
Finally, hypertension is a common co-morbidity in both CVD and T2DM. A substantial proportion of people with T2DM have concomitant hypertension (20–60%, depending on age, sex, ethnicity and body mass index). Hypertension almost doubles the risk of all-cause mortality, and increases the risk of coronary artery disease threefold [6, 7]. In addition, hypertension enhances the progression of diabetic complications such as nephropathy, retinopathy and neuropathy.
Lowering of BP, using appropriate antihypertensive treatment, reduces the risk of complications in T2DM patients [8–10]. The 8th Joint National Committee (JNC) recommendations for the management of hypertension in adults suggest targets of <140 mmHg for systolic BP (SBP) and <85–90 mmHg for diastolic BP (DBP) in patients with T2DM [11]. However, hypertension associated with T2DM is especially challenging to treat [12], with most T2DM patients needing more than one drug for BP control. A systematic survey of general practitioners in Canada revealed that 28–39% of hypertensive patients with underlying T2DM failed to achieve the BP targets recommended by the current JNC guidelines [13, 14]. New and improved treatment options for BP lowering are urgently needed to improve CV prognosis among patients with T2DM and hypertension.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of drugs that have been extensively investigated for the treatment of hyperglycemia in T2DM. These drugs reduce hyperglycemia by blocking renal glucose reabsorption, thereby promoting increased renal excretion of glucose [15]. Over and above glycemic control, these drugs have shown benefit with respect to a number of CV risk factors [16, 17]. The present review discusses the potential role of SGLT2 inhibitors in addressing risk factors and complications associated with T2DM, such as hypertension, from a cardiology perspective. It also discusses the potential use of SGLT2 in acute and chronic CV syndromes and obesity.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
SGLT2 Inhibitors
The United States Food and Drug Administration and the European Medicines Agency have approved 3 drugs in this class: canagliflozin, dapagliflozinand empagliflozin. A number of other members of this class are in clinical development. A large meta-analysis of data from 58 trials of SGLT2 inhibitors (dapagliflozin or canagliflozin) concluded that SGLT2 inhibitors have a statistically significant favorable effect on HbA1c [17]. This effect was consistently observed in T2DM patients on background treatment with a range of antihyperglycemic agents, including glipizide and insulin [18]. Similar beneficial effects were reported with empagliflozin; in patients who were uncontrolled with metformin, the placebo-adjusted mean decrease in HbA1c was 0.57% with 10 mg empagliflozin and 0.64% with 25 mg after 24 weeks of treatment [19].
The available safety data indicate that SGLT2 inhibitors are generally well tolerated [20, 21]. Hypoglycemia is less common than with many other classes of antihyperglycemic agents, since the mechanism of action is independent of insulin. Urinary tract infections and genital infections (mainly fungal) are more commonly reported, but these are generally mild in nature and can be easily treated [22, 23]. Volume depletion, another common side effect reported with SGLT2 inhibitors, can occur as a result of the osmotic diuresis associated with SGLT2 inhibitor-induced glycosuria. Other side effects/symptoms are frequent urination, thirst and, potentially, orthostatic hypotension [20]. However, these are not clinically significant, and rarely lead to discontinuation of therapy.
Effects on Hemodynamics
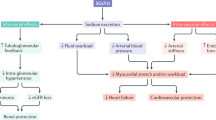
The favorable effects of SGLT2 inhibitors on a number of conventional CV risk factors, such as BP, blood glucose levels and body weight, are well documented (Fig. 1). Clinical studies are under way to investigate other effects of these drugs on other CV risk factors and outcomes [24].
In particular, the osmotic diuresis effects of SGLT2 inhibitors have received considerable attention. Along with blocking glucose reabsorption, SGLT2 inhibitors also reduce protein and sodium reabsorption in the nephron, resulting in a mild osmotic diuresis [25]. The loss of volume and sodium activates the renin-angiotensin-aldosterone (RAAS) system and initiates a counter-regulatory compensatory mechanism by the kidneys to maintain sodium homeostasis [26] (Fig. 1). In addition, weight loss resulting from glycosuria and the associated net caloric loss may further contribute to BP lowering.
Preload Reduction
Because of their intriguing effects on renal hemodynamics, it has been suggested that SGLT2 inhibitors could have a role in treating T2DM patients with both types of chronic heart failure (HF), namely reduced ejection fraction HF (HFrEF) or preserved ejection fraction HF (HFpEF). HFrEF is characterized by a left ventricular ejection fraction of ≤50%, increased left ventricular mass, as well as increased end-diastolic and end-systolic volume. This is typically seen in diabetic cardiomyopathy, valval heart diseases and cardiomyopathy. In contrast, HFpEF is characterized by a left ventricular ejection fraction of >50%, increased left ventricular mass, unchanged or decreased end-diastolic and end-systolic volume. This type of HF is seen in restrictive cardiomyopathy, hypertensive heart disease, and hypertrophic obstructive cardiomyopathy. SGLT2 inhibitor-induced diuresis would be expected to result in preload reduction (Fig. 1), which may be beneficial in chronic HF patients with a reduced ejection fraction. However, care should be taken when using diuretics in such patients, due to the possibility of excessive preload reduction [27].
Afterload Reduction
There is evidence that SGLT2 inhibitors may reduce afterload as well as preload. Cherney et al. studied hemodynamic changes in type 1 diabetes mellitus patients who were treated with empagliflozin 25 mg daily for 8 weeks. This study documented reductions in BP, arterial stiffness and sympathetic nervous system activity. Radial artery and carotid waveforms, augmentation index (AIx), heart rate, and aortic pulse wave velocity were measured. The AIx is an indicator of central aortic pressure enhancement by a reflected pulse wave and is used as a predictor of adverse CV events. AIx is a ratio calculated using BP waveforms; the greater the augmentation or enhancement, the greater the degree of arterial stiffness. After 8 weeks of treatment, AIx was significantly reduced in the empagliflozin group, compared with the placebo group, suggesting reductions in the afterload as well as the preload (Fig. 1). No significant changes in sympathetic nervous system activity were reported [28], further suggesting that SGLT2 inhibitors reduce afterload.
SGLT2 inhibitors have also been reported to reduce the levels of plasma uric acid. This is thought to be due to their effects on a urate transporter, solute carrier family 2, facilitated glucose transporter member 9, which transports urate into the urine in exchange for glucose [18].
Effects on BP
Almost all SGLT2 inhibitor studies have reported significant BP reductions, with a larger effect on SBP (1.66–6.90 mmHg) than on DBP (0.88–6.99 mmHg) (Fig. 1). A pooled analysis of 4 Phase III, placebo-controlled clinical studies indicated modest reductions in SBP with canagliflozin (−3.3 and −4.5 mmHg with the 100 and 300 mg doses, respectively) [29]. Similarly, analysis of pooled data from 4 Phase III empagliflozin clinical trials revealed significant placebo-adjusted reductions in SBP with empagliflozin treatment (10 or 25 mg once daily for 24 weeks as a monotherapy or add-on therapy to metformin, metformin plus sulfonylurea, or pioglitazone with or without metformin) [30]. There are reports that dapagliflozin had effects on reducing the BP. In a study, which compared dapagliflozin as monotherapy in various dosing schedule vs. placebo in T2DM patients, reported reduction in office SBP and DBP in those patients up to −5.7 and 3.3 mmHg, respectively. Few studies had explored the efficacy of this drug as an add-on to the oral antidiabetics. In a study consisting of over 800 patients with T2DM who were on stable insulin dose with or without other oral antidiabetics, on add on dapagloflozin or placebo, the researchers reported reductions in SBP with dapagliflozin (mean change: −1.49 mmHg in the placebo group vs −5.30, −4.33, and −4.09 mmHg in the groups receiving 2.5, 5, and 10 mg of dapagliflozin, respectively) and non-significant DBP reductions (−1.31 with placebo vs −2.96, −2.64, and −2.85 mmHg in the groups who received 2.5, 5, and 10 mg of dapagliflozin).
Effect on Pulse
There is no increase in pulse rate with SGLT2 inhibitors. A 104-week outcome study of canagliflozin indicated that the 100 and 300 mg dose reduced SBP and DBP compared with glimepiride, with no notable changes in pulse rate [31]. This contrasts with the tachycardic reported with use of other glucose lowering drugs such as glucagon like peptide 1 receptor agonists.
In the short term, these BP-lowering effects have been attributed to both preload and afterload reduction. This is a unique feature of SGLT2 inhibitors, which needs to be highlighted in cardiology circles. Long-term effects may be mediated through RAAS activation and/or reductions in body weight [26].
Effects on Body Weight
Reductions in body weight are consistently observed with SGLT2 inhibitor treatment (Fig. 1). This effect may be attributed to diuresis or volume depletion, as well as to net loss of calories in the form of glucose. Among T2DM patients inadequately controlled with metformin, weight loss occurred with dapagliflozin treatment, compared with weight gain on glipizide (−3.2 vs. +1.4 kg over 52 weeks of treatment, dapagliflozin vs. glipizide; P < 0.0001) [31]. Similar observations have been made regarding canagliflozin treatment. In T2DM patients inadequately controlled with metformin and sulfonylureas, reductions of −1.9 and −2.5 kg were achieved with 100 and 300 mg canagliflozin, vs. −0.8 kg with placebo [30, 32]. Weight loss with SGLT2 inhibitors was also documented in patients who were taking a combination of oral antidiabetic drugs and insulin (P < 0.001). The sustained impact on body weight is thought to be the result of fat loss.
Effects on Lipid Profiles
A small number of studies have reported beneficial effects of SGLT2 inhibitors on lipid parameters, including reductions in triglyceride levels and small increases in high-density lipoprotein cholesterol (HDL-C) levels [14, 33, 34] (Fig. 1). Increases in total cholesterol and low-density lipoprotein cholesterol levels have also been reported. A study of T2DM patients who were uncontrolled with metformin (>1500 mg/day) reported an increase in HDL-C and a reduction in triglycerides with dapagliflozin compared with placebo [18]. However, the clinical significance of these changes in lipid parameters is not yet known.
Effects on CV Outcomes
Although SGLT2 inhibitor treatment appears to improve a number of classical CV risk factors such as BP, body weight and HbA1c in the short term, the long-term benefits (e.g. sustained reductions in risk factors) have yet to be ascertained. To date, only a handful of studies have reported CV outcomes, as these were generally not planned as primary outcomes in the pivotal SGLT2 inhibitor trials [33].
In the absence of long-term real-world data, a modeling study by Dziuba et al. [35] investigated the effects of adding dapagliflozin to standard therapy in a simulated T2DM population. Based on modeling, a greater decrease in CV and microvascular complications was predicted for dapagliflozin added to standard therapy, compared with standard therapy alone [35].
Subsequently, the effects of empagliflozin on CV outcomes were investigated in a large multi-country study of over 7000 T2DM patients with established CVD [36]. This study was a randomized, double-blind, placebo-controlled trial to investigate the effects of empagliflozin (10 or 25 mg added to standard care) on CV events in patients with T2DM who were at high risk of CV events. The primary outcome was a composite of death from CV causes, nonfatal myocardial infarction (excluding silent myocardial infarction), or nonfatal stroke; the key secondary outcome was a composite of the primary outcome plus hospitalization for unstable angina. High-risk T2DM patients who received empagliflozin plus standard care had significantly lower rates of the primary composite outcome and of death from any cause than the placebo group (standard care only) [35].
The Canagliflozin Cardiovascular Assessment Study (CANVAS) trial (ClinicalTrials.gov identifier: NCT01032629) is among several ongoing trials for which interim analysis results have been published. In this analysis, 201 4-point major adverse cardiac events (MACE) occurred in over 9000 patients with a hazard ration of 1.0 (95% CI: 0.72, 1.39).This study will continue until 420 3-point events have been accrued, and will be evaluated for the potential CV benefits. This study started in 2009 and is expected to conclude the final results in 2017. One of the largest ongoing trials, DECLARE-TIMI58 (Dapagliflozin Effect on CardiovascuLAR Events) (ClinicalTrials.gov identifier: NCT01730534) is a multi- national randomized, double blind, placebo controlled Phase IIIB superiority trial designed to test the hypothesis that patients with T2DM, who were on long term dapaglifolzin, will have a reduced incidence of the composite endpoint of CV death, myocardial infarction or ischemic stroke. This study will also seek to exclude the unacceptable CV risk from the drug in these patients. The teams have plans to enroll over 17,000 participants with T2DM and have indicated a target of 1390 3-point MACE. The results are expected to be out in 2018, with a hope to see if dapagliflozin could offer CV benefits as well as addressing safety related issues.
In a recently published study of the EMPA-REG OUTCOME trial (ClinicalTrials.gov identifier: NCT01131676), it was found that in patients with T2DM who were at high CV risk, empagliflozin was effective in slowing the progression of kidney disease. With an aim to assess the long term renal effects of empagliflozin, a secondary analysis of the pre-specified component of the secondary microvascular outcome of the trial was performed. The study reported a lower incidence or worsening nephropathy in the Empagliflozin arm (12.7%) when compared with the placebo (18.8%). There were raised serum creatinine levels in 1.5% of patients in the empagliflozin group versus 2.6% in the placebo group, which was a significant reduction of the relative risk. The need for renal replacement therapy was lower in the empagliflozin group than the placebo. The study concluded that the use of empagliflozin in patients with T2DM at high CV risk was associated with slower progression of kidney disease compared with the placebo [37].
Conclusion
The safety and efficacy of SGLT2 inhibitors (including canagliflozin, dapagliflozin and empagliflozin) for the treatment of hyperglycemia in T2DM has been well documented. For now, these drugs have proven to be a useful addition to the diabetes treatment arsenal, given their beneficial effects on CV risk factors. Looking to the future, the results of planned and ongoing trials to investigate long-term safety and efficacy of SGLT2 inhibitors, especially in terms of CV outcomes, will be of enormous interest. These results may provide additional motivation for investigating the use of SGLT2 inhibitors in the treatment of a broader range of CV-related conditions.
References
ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–38.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14.
World Health Organization (WHO). Cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/. Accessed 15 Feb 2016.
Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70.
Aylsworth A, Dean Z, VanNorman C, Okere AN. Dapagliflozin for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2014;48(9):1202–8.
Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease an update. Hypertension. 2001;37(4):1053–9.
Adeniyi OV, Yogeswaran P, Longo-Mbenza B, Goon DT. Uncontrolled hypertension and its determinants in patients with concomitant type 2 diabetes mellitus in rural South Africa. PLoS One. 2016;11(3):e0150033.
American Diabetes Association. Standards of medical care in diabetes—cardiovascular disease and risk management. Diabetes Care. 2015;39(Supplement 1):S60–71.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357.
Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD—summary. Diabetes Vasc Dis Res. 2014;11(3):133–73.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Williams B. Resistant hypertension: an unmet treatment need. Lancet. 2009;374(9699):1396–8.
Nelson SA, Dresser GK, Vandervoort MK, Wong CJ, Feagan BG, Mahon JL, et al. Barriers to blood pressure control: a STITCH substudy. J Clin Hypertens. (Greenwich). 2011;13(2):73–80.
Casagrande SS, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–9.
Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diabetes Vasc Dis Res. 2015;12(2):78–89.
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12(2):90–100.
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–74.
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–33.
Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–9.
Boyle LD, Wilding JP. A safety evaluation of canagliflozin: a first-in-class treatment for type 2 diabetes. Expert Opin Drug Saf. 2014;13(11):1535–44.
Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457–66.
Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complicat. 2013;27(5):473–8.
Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med. 2013;125(3):33–46.
In:ClinicalTrials.gov[Internet].Bethesda (MD). https://clinicaltrials.gov/ct2/results?term=SGLT2+inhibitors&pg=2. Accessed 10 Feb 2016.
List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;79(120):S20–7.
Peene B, Benhalima K. Sodium glucose transporterprotein2 ihibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab. 2014;5:124–36.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97.
Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13(1):28.
Hach T, Gerich J, Salsali A, Kim G, Hantel S, Woerle HJ, et al. Empagliflozin improves glycemic parameters and cardiovascular risk factors in patients with Type 2 Diabetes (T2DM): Pooled data from four pivotal phase III trials. Diabetologie und Stoffwechsel. 2014;9:142.
Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–82.
Weir MR, Januszewicz A, Gilbert RE, Lavalle Gonzalez FJ, Meininger G. Lower blood pressure (BP) with canagliflozin (cana) in subjects with type 2 diabetes mellitus (T2DM). Can J Diabetes. 2013;37S4:S3. doi:10.1016/j.jcjd.2013.08.005.
Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015–22.
Hardy E, Ptasynska A, de Bruin TWA, Johnsson E, Parikh SJ, List J, et al. Changes in lipid profiles of patients with type 2 diabetes mellitus on dapagliflozin therapy. Diabetologie und Stoffwechsel. 2014;9(S 01):947.
Desouza CV, Gupta N, Patel A. Cardiometabolic effects of a new class of antidiabetic agents. Clin Ther. 2015;37(6):1178–94.
Dziuba J, Alperin P, Racketa J, Iloeje U, Goswami D, Hardy E, et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014;16(7):628–35.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi:10.1056/NEJMoa1515920.
Acknowledgments
Sponsorship and article processing charges for this study were funded by AstraZeneca Pharma India Ltd, Bangalore, India. The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole, and has given final approval for the version to be published.
Disclosures
Sanjay Kalra has no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CBE4F06021C48730.
An erratum to this article is available at http://dx.doi.org/10.1007/s40119-016-0070-6.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kalra, S. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors and Cardiovascular Disease: A Systematic Review. Cardiol Ther 5, 161–168 (2016). https://doi.org/10.1007/s40119-016-0069-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-016-0069-z