Abstract
Introduction
The objective of this study was to investigate the effect of adding exenatide to continuous subcutaneous insulin infusion (CSII) therapy on the precise insulin doses required by type 2 diabetic patients to maintain glycemic control.
Methods
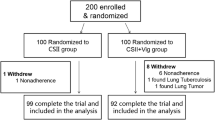
This was a single-center, randomized, controlled, open-label trial. Uncontrolled T2D patients were recruited between March 2010 and November 2011 at Nanjing First Hospital, China. Subjects were randomly assigned (1:1) to either an exenatide add-on to CSII group or a CSII therapy only (i.e., control) group (n = 18, respectively) for 5 weeks. Patients were subjected to 3 days of continuous glucose monitoring (CGM) during the screening period and after therapy. The precise insulin doses, the times taken by the patients to achieve euglycemic control, and the mean amplitude of glycemic excursion (MAGE) at the endpoint were compared between the two groups. The primary endpoint was precise insulin dose differences between groups from baseline to the endpoint.
Results
A total of 36 subjects were admitted as inpatients. Patients in the exenatide add-on therapy group needed less insulin titration time to achieve glycemic control (3.67 ± 1.33 vs. 4.78 ± 1.00 days, P = 0.028) and significantly lower bolus insulin doses than the control group at the endpoint (total bolus, 0.13 ± 0.03 vs. 0.17 ± 0.04 U/kg, P = 0.02, breakfast bolus, 0.05 ± 0.01 vs. 0.06 ± 0.01 U/kg, P = 0.01, lunch bolus, 0.04 ± 0.01 vs. 0.06 ± 0.01 U/kg, P = 0.01, dinner bolus, 0.04 ± 0.01 vs. 0.05 ± 0.01 U/kg, P = 0.01, respectively). Moreover, the CGM data showed that patients in the exenatide add-on therapy group exhibited a significant reduction in MAGE as compared to the control group (2.96 ± 1.14 vs. 4.21 ± 1.39 mmol/L, P = 0.012).
Conclusion
Our data suggest that adding exenatide therapy to CSII therapy leads to an improvement in glycemic excursions and the use of smaller bolus insulin doses.
Trial Registration
Chinese Clinical Trial Registry identifier, ChiCTR-PPR-15007045.
Similar content being viewed by others
Introduction
Uncontrolled type 2 diabetes (T2D) is associated with long-term microvascular and cardiovascular complications that are dangerous or even fatal. Euglycemic control is not achieved in patients who require insulin. Intensive insulin therapy is commonly employed in patients with T2D to keep their blood glucose levels within the target range. Intensive insulin therapy consists of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI). Short-term intensive insulin therapy improves blood glycemic control, which is accompanied by the recovery of β-cell function in people with T2D [1,2,3,4,5]. We recently observed that patients with newly diagnosed or longstanding T2D treated with CSII therapy presented a greater improvement in mean amplitude of glycemic excursion (MAGE), as detected by continuous glucose monitoring (CGM) [6]. Dramatic blood glycemic excursions may be an independent risk factor for cardiovascular disease in patients with onset T2D [7, 8]. Large glucose fluctuations may lead to the overproduction of superoxide by the mitochondrial electron-transport chain, which induces nitrosative stress [9].
Exenatide, a glucagon-like peptide-1 receptor agonist (GLP-1RA) that acts as a blood-glucose-lowering agent, is approved as second-line treatment for patients with T2D to achieve euglycemic control [10, 11]. By activating the GLP-1 receptor, exenatide increases insulin secretion and decreases glucagon secretion. Furthermore, exenatide reduces food intake and lowers gastric emptying [10, 12, 13]. An evidence-based review showed that exenatide provides various benefits for patients with T2D, including A1C reduction, weight loss, and minimization of the risk of hypoglycemia [13]. In addition, exenatide shows the ability to improve blood glycemic fluctuations. Exenatide therapy in subjects with T2D led to an improvement in glycemic variability (MAGE, as monitored by CGM) as compared with that obtained with glimepiride therapy [14]. A multicenter, open-label, randomized, parallel trial performed in China found that patients treated with exenatide add-on metformin therapy exhibited statistically significant and clinically relevant reductions in glucose variability compared with those on metformin-based biphasic insulin aspart 30 therapy only [15]. A 26-week study demonstrated that adding exenatide therapy to insulin glargine and metformin results in improved coefficients of glucose variation compared with those achieved with insulin glargine and metformin plus rapid-acting insulin [16].
Patients with poorly controlled T2D who were treated with exenatide add-on CSII therapy showed significantly improved glucose control as measured by the fingerstick test [17], although the glycemic profile obtained from intermittent fingerpricks has limitations [18]. Intermittent fingerprick tests usually include three fasting capillary blood glucose measurements and capillary blood glucose measurements performed 2 h after each of three meals [5]. Thus, 24-h blood glycemic excursions are undoubtedly missed when these point-to-point glimpses of blood glucose are obtained. CGM provides a unique opportunity to examine the 24-h glucose excursions in T2D patients who have achieved euglycemic control. However, little is known about the effect of adding exenatide to CSII therapy on the precise insulin doses required by T2D patients to maintain glycemic control. We therefore performed a single-center, randomized, controlled, open-label trial using CGM to assess blood glucose fluctuations in T2D patients treated with exenatide add-on therapy to CSII.
Methods
This was a single-center, randomized, controlled, open-label trial. Between March 2010 and November 2011, a total of 36 patients with uncontrolled T2D were recruited at Nanjing First Hospital, Nanjing Medical University, China. The inclusion criteria were (1) age between 18 and 80 years; (2) 7.5% ≤ HbA1c ≤ 12% at screening; (3) confirmed type 2 diabetes for at least half a year; (4) body mass index 21–35 kg/m2. Patients were excluded from the analysis if they had ketoacidosis, chronic kidney disease, were positive for antiglutamic acid decarboxylase (aGAD) antibody, or if they had maturity-onset diabetes in the young (MODY) or mitochondrial diabetes mellitus [5]. Patients with known cancers or known allergies to insulin were excluded [5, 19].
All procedures followed were in accordance with the ethical standards of Nanjing First Hospital and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients before they were included in the study.
The trial included a 4-day screening period to measure blood glucose profiles, as monitored by CGMS, and a 5-week treatment period. During the screening period, the subjects were admitted as inpatients to collect baseline parameter values and for 3 days of retrospective CGM (Medtronic Incorporated, Northridge, CA, USA), which was performed as previously described [6, 20]. After the CGM data had been collected, the enrolled subjects were randomized in equal numbers to a CSII-only regimen or to an exenatide add-on to CSII regimen. For the first 2 weeks, the dose was 5 μg exenatide (AstraZeneca, Cambridge, UK) 60 min before breakfast and before dinner, which was then titrated up to a standard dose of 10 μg twice a day until the completion of the study.
The total daily insulin (Aspart, Novo Nordisk, Bagsværd, Denmark) dose was 0.5 IU/kg, which was given in two injection modes: one-third of the total daily dose was given as equal boluses at three meals; the remaining insulin was given as a basal dose. Investigators titrated insulin doses on an individual-patient basis according to the titration algorithm (if the fasting blood glucose level was less than 4.4 mmol/L, the basal insulin dose was reduced by 2 units; if the fasting blood glucose level was within 4.4–6.1 mmol/L, the basal insulin dose was unchanged; if the fasting blood glucose level was within 6.2–7.8, 7.9–10.0, or >10.0 mmol/L, the basal insulin dose was subsequently increased by 2, 4, and 6 units, respectively, and if the postprandial blood glucose level was up, the bolus insulin dose was titrated according to the same algorithm used for the basal dose), as we described previously [6]. After 5 weeks of therapy, the patients were admitted as inpatients to perform retrospective CGM for 3 days. All subjects were instructed to maintain a similar level of physical activity and they received a similar diet and a similar level of carbohydrates during the two CGM periods. In addition, the serum glycated albumin (GA) concentration was measured using liquid enzymatic assays (Lucica GA-L; Asahi Kasei Pharma, Tokyo, Japan) at baseline and the endpoint, as described previously [21].
The treatment period required to achieve euglycemic control (the fasting capillary blood glucose was less than 6.1 mmol/L and the capillary blood glucose at 2 h after each of three meals was less than 8.0 mmol/L) was recorded for each subject [5, 22]. Changes in insulin dose and body weight following treatment were also analyzed. The 24-h mean blood glucose (MBG), 24-h MAGE, and the incremental areas under the curve (AUC) of plasma glucose >10.0 mmol/L and <3.9 mmol/L were calculated by software provided by Medtronic Inc., and hypoglycemic episodes were also recorded. MAGE was calculated for each patient by measuring the arithmetic mean of the ascending and descending excursions between consecutive peaks and nadirs during the same 24-h period; only absolute excursion values >1 SD were considered, as described previously [6, 20].
The primary endpoint was the precise insulin dose changes before and after therapies between groups. Secondary endpoints were the changes in MAGE and body weight from baseline to the completion of treatment. The hourly mean blood glucose concentrations, the 24-h MBG, and the AUCs of hypoglycemia and hyperglycemia were also analyzed.
This study was registered with ClinicalTrials.gov, number ChiCTR-PPR-15007045. http://www.chictr.org.cn/showproj.aspx?proj=8321.
Statistical Analysis
Statistical analysis was performed using the SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to assess the distribution of data. Values of normally distributed and continuous variables are presented here as mean (standard deviation, SD). The mixed ANOVA model (2 × 2) test was used to compare differences within groups. The two-way ANOVA was used for comparisons between groups. Bonferroni correction was performed. P values were two-tailed with a significance level of 5%.
Results
Baseline Characteristics
A total of 36 patients were consecutively recruited to the study and randomized to the exenatide add-on to CSII therapy group (n = 18) or the CSII therapy group (n = 18). In terms of mean values, the patients were 52.57 ± 9.91 years of age, had been diabetic for 11.98 ± 7.83 years, and had a body-mass index of 26.36 ± 3.17 kg/m2, HbA1c of 9.61 ± 1.60%, mean fasting blood glucose of 11.21 ± 2.97 mmol/L, mean fasting plasma insulin of 9.75 ± 8.88 μU/mL, and mean fasting plasma C-peptide of 2.64 ± 0.96 mmol/L. There were no significant demographic differences within groups at baseline (Table 1). All patients finished the study.
Glucose, Insulin, GA, and Body Weight Profiles
Patients in the exenatide add-on group group reached their glycemic goals more quickly than the control group did (3.67 ± 1.33 vs. 4.78 ± 1.00 days, P = 0.028). The daily total insulin dose required by subjects to maintain euglycemic control in the exenatide add-on therapy group was significantly lower than that required by the control group at the endpoint (0.28 ± 0.06 U/kg/d vs. 0.33 ± 0.07 U/kg/d, P = 0.02). We compared the bolus and basal insulin doses used by patients between groups. Our data showed that patients in the exenatide add-on group needed significantly lower bolus insulin doses than those in the control group (total bolus, 0.13 ± 0.03 vs. 0.17 ± 0.04 U/kg, P = 0.02, breakfast bolus, 0.05 ± 0.01 vs. 0.06 ± 0.01 U/kg, P = 0.01, lunch bolus, 0.04 ± 0.01 vs. 0.06 ± 0.01 U/kg, P = 0.01, dinner bolus, 0.04 ± 0.01 vs. 0.05 ± 0.01 U/kg, P = 0.01, respectively). We observed a significant difference in basal insulin dose between the exenatide add-on group and the control group (0.15 ± 0.04 vs. 0.17 ± 0.06 U/kg, P = 0.10). The serum GA concentration was significantly reduced at the endpoint in both groups (exenatide add-on therapy group: 25.76 ± 15.99% vs. 38.87 ± 7.06%, P = 0.00, and control group: 25.86 ± 19.57% vs. 42.04 ± 12.39%, P = 0.00), but we did not observe any difference between the GA levels of the two groups at the endpoint (P = 0.76). Subject body weight was significantly reduced in the exenatide add-on therapy group from baseline to the completion of treatment (from 75.78 ± 5.14 to 71.33 ± 5.17 kg, P = 0.000) (Table 2). Moreover, the reduction in patient body weight in the exenatide add-on group was significantly higher than that in the control group (4.44 ± 2.31 vs. 2.5 ± 1.18 kg, P = 0.02).
Glycemic Fluctuation Profiles
There were no differences in 24-h MBG within (exenatide add-on group 7.06 ± 1.04 vs. control group 7.62 ± 2.09 mmol/L, P = 0.351). We did not observe any significant difference between the exenatide add-on group and the control group in hourly mean blood glucose concentration (Fig. 1) or incremental AUC > 10 mmol/L (0.10 ± 0.17 vs. 0.40 ± 1.02 mmol/L day, P = 0.624) at the endpoint (Table 3). However, CGM data showed that subjects in the exenatide add-on group had a lower MAGE (2.96 ± 1.14 vs. 4.21 ± 1.39 mmol/L, P = 0.012) than those in the control group at the endpoint (Table 3).
Safety and Tolerance
We also compared the incremental AUC < 3.9 mmol/L between the two groups. Exenatide add-on therapy did not increase hypoglycemic episodes (0.01 ± 0.03 vs. 0.02 ± 0.06 mmol/L day, P = 0.624) (Table 3). No episodes of hypoglycemia requiring medical assistance were reported in either group. All therapies were well tolerated by the subjects during the study, and no adverse events were reported in the groups.
Discussion
We conducted a prospective study of patients with uncontrolled T2D and demonstrated that exenatide add-on to CSII therapy could significantly reduce the bolus insulin doses needed and facilitate further improvements in blood glycemic fluctuations. We also found that patients treated with combined exenatide and CSII therapy needed a shorter treatment period to achieve euglycemic control than those in the control (CSII) group at the endpoint, and that combination therapy was associated with minimal hypoglycemia and weight loss.
Exenatide used as either monotherapy or in combination with other therapies has been shown to produce significant reductions in A1C and improved levels of fasting plasma glucose (FPG) [13, 23,24,25]. In accordance with the reduced FPG levels, our CGM data indicated that exenatide add-on therapy to CSII prompted further improvement in blood glycemic excursions (i.e., in MAGE) compared with CSII alone. A study using CGM indicated that patients treated with exenatide for 16 weeks lowered their total daily mean glucose, SD, and MAGE, whereas those on glimepiride did not [14]. Exenatide in combination with metformin therapy resulted in a significant reduction in glucose excursions from baseline to endpoint in patients with T2D [15]. A 26-week study demonstrated that the addition of exenatide therapy to insulin glargine and metformin resulted in a better coefficient of glucose variation than that obtained with insulin glargine and metformin plus rapid-acting insulin [16]. A previous study demonstrated that, compared with CSII therapy alone, the addition of twice-daily exenatide to CSII in patients with poorly controlled T2D significantly improved glucose control, as monitored by the fingerstick test [17]. In comparison with long-acting GLP-1 RAs, exenatide—the first short-acting GLP-1 receptor agonist (RA) to be approved for use to smooth blood glucose concentrations in patients with T2D—represents a potential treatment for patients who predominantly suffer from postprandial hyperglycemia rather than fasting hyperglycemia [26]. However, long- and short-acting GLP-1 RAs are useful when employed as an add-on therapy for patients who are at risk of hypoglycemia or overweight [26]. In the present trial, we used CGM to assess the blood glucose fluctuations in T2D patients treated with exenatide add-on therapy to CSII. Our CGM data demonstrated that there was no statistically significant difference within groups in the 24-h MBG or in the incremental AUC < 3.9 mmol/L at the endpoint. Subjects in the exenatide plus CSII group had a lower MAGE and incremental AUC > 10 mmol/L at the endpoint compared with the control group. Our data led us to a somewhat different conclusion to a previous study which noted a significantly higher standard deviation of plasma glucose in patients with poorly controlled T2D who were treated with exenatide add-on intensive insulin therapy with CSII [17]. However, the glycemic profile obtained from intermittent fingerpricks has its limitations [18]. Intermittent fingerprick tests usually involve three fasting capillary blood glucose measurements and capillary blood glucose measurements taken 2 h after each of three meals [5]. Thus, 24-h blood glycemic excursions are undoubtedly missed by these point-to-point glimpses of blood glucose. CGM provides a unique opportunity to examine 24-h glucose excursions in T2D patients who have achieved euglycemic control.
Whether exenatide add-on therapy can reduce the insulin doses required by patients to achieve glycemic control is still the focus of debate. In some studies, some patients were able to reduce their insulin doses, especially their bolus insulin doses, after the initiation of a GLP-1 RA [27]. For patients with A1C ≤8.0% at randomization who were treated with exenatide and insulin combination therapy, the insulin dose was reduced by 20% [28]. However, a study performed by Lin and colleagues indicated that the insulin dose remained unchanged within groups (exenatide add-on intensive insulin therapy with CSII vs. CSII therapy only), even at the endpoint, in patients with poorly controlled T2D [17]. Our data indicated that exenatide add-on therapy to CSII led to a discernable but not statistically significant insulin dose reduction after 5 weeks of treatment. However, treatment with the exenatide add-on shortened the time taken by patients to achieve glycemic control, thus confirming the ability of exenatide to improve blood glycemic fluctuations [29].
Insulin therapy confers an increased risk of hypoglycemia and weight gain [30,31,32]. Exenatide treatment provides a unique opportunity to avoid the risk of hypoglycemia and weight gain during CSII treatment [13]. Addition of a GLP-1 agonist to basal insulin treatment has the potential to improve glycemic control without increasing the risk of hypoglycemia and weight gain, even in patients with longstanding T2D. However, the long-term durability of this combination therapy requires further investigation [33]. CSII provides precise insulin delivery throughout the day and accurately simulates the function of the islet cells. CSII therapy is regarded as a safe and valuable alternative in patients with new-onset or longstanding T2DM [2,3,4,5]. The addition of exenatide to CSII therapy may provide a unique opportunity to smooth glycemic variations without incurring an increased risk of hypoglycemia and weight gain. Our data showed that patients in the exenatide add-on therapy group experienced weight loss with a minimal risk of hypoglycemia under strictly controlled conditions in the hospital setting. However, future studies are needed to identify the long-term effects of this add-on therapy. Compared with some conventional therapies, GLP-1RAs present the advantages of reducing appetite and increasing satiety [10, 11, 34]. Even when used in combination with insulin therapy, these benefits were sustained for up to 3 years [35]. Furthermore, in addition to the weight loss, exenatide therapy also has a favorable effect on liver and pericardial fat contents in obese patients with T2D [36]. It is well documented that exenatide add-on therapy does not increase the risk of hypoglycemia [28, 37,38,39]. In this trial, we did not observe any increase in the risk of hypoglycemia from baseline to the endpoint for each group.
However, GLP-1 RAs show a greater potential to lower HbA1c in Asian populations than in non-Asian populations [40]. Potential mechanisms for this difference between populations may be that Asian T2D populations have lower BMIs and slimmer waists than their Western counterparts [41], and also that they have different nutrient intake patterns and life styles [42].
Our study also has other limitations: firstly, the unblended nature of the study design, which was open to bias; secondly, the sample size was relatively modest; thirdly, we did not observe for a long time period.
Conclusion
In conclusion, exenatide therapy as an add-on to intensive insulin therapy with CSII has the ability to reduce the bolus insulin doses needed and to further improve blood glycemic control. Patients with T2D who were treated with the exenatide add-on therapy required less time to achieve euglycemic control and showed increased weight loss.
References
Retnakaran R, Drucker DJ. Intensive insulin therapy in newly diagnosed type 2 diabetes. Lancet. 2008;371:1725–6.
Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–6.
Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–602.
Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–32.
Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60.
Li FF, Fu LY, Zhang WL, Su XF, Wu JD, Sun J, et al. Blood glucose fluctuations in type 2 diabetes patients treated with multiple daily injections. J Diabetes Res. 2016;2016:1028945.
Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40.
DECODE Study Group, European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality. comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Gallwitz B. Preclinical and clinical data on extraglycemic effects of GLP-1 receptor agonists. Rev Diabetic Stud: RDS. 2009;6:247–59.
Peng H, Want LL, Aroda VR. Safety and tolerability of glucagon-like peptide-1 receptor agonists utilizing data from the exenatide clinical trial development program. Curr Diab Rep. 2016;16:44.
Irace C, Fiorentino R, Carallo C, Scavelli F, Gnasso A. Exenatide improves glycemic variability assessed by continuous glucose monitoring in subjects with type 2 diabetes. Diabetes Technol Ther. 2011;13:1261–3.
Xu S, Liu X, Ming J, Ji Q. Comparison of exenatide with biphasic insulin aspart 30 on glucose variability in type 2 diabetes: study protocol for a randomized controlled trial. Trials. 2016;17:160.
FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care 2016;39:973–1.
Lin CH, Hsieh SH, Sun JH, Tsai JS, Huang YY. Glucose variability and beta- cell response by GLP-1 analogue added-on CSII for patients with poorly controlled type 2 diabetes. Sci Rep. 2015;5:16968.
Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–62.
Ziegler R, Tubili C, Chico A, Guerci B, Lundberg E, Borchert M, et al. ProAct study: new features of insulin pumps improve diabetes management and glycemic control in patients after transition of continuous subcutaneous insulin infusion systems. Diabetes Technol Ther. 2013;15:738–43.
Li FF, Xu XH, Fu LY, Su XF, Wu JD, Lu CF, et al. Influence of acarbose on plasma glucose fluctuations in insulin-treated patients with type 2 diabetes: a pilot study. Int J Endocrinol. 2015;2015:903524.
Zhou Q, Shi DB, Lv LY. The establishment of biological reference intervals of nontraditional glycemic markers in a Chinese population. J Clin Lab Anal. 2016. doi:10.1002/jcla.22097.
Schnell O, Mertes G, Standl E. Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes Obes Metab. 2007;9:853–8.
Nikfar S, Abdollahi M, Salari P. The efficacy and tolerability of exenatide in comparison to placebo; a systematic review and meta-analysis of randomized clinical trials. J Pharm Pharm Sci. 2012;15:1–30.
Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206.
Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology. 2010;86:44–57.
Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther. 2015;6:239–56.
Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36:893–905.
Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–12.
Wu JD, Xu XH, Zhu J, Ding B, Du TX, Gao G, et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:143–8.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Charbonnel B, Cariou B. Pharmacological management of type 2 diabetes: the potential of incretin-based therapies. Diabetes Obes Metab. 2011;13:99–117.
Barnett AH, Cradock S, Fisher M, Hall G, Hughes E, Middleton A. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int J Clin Pract. 2010;64:1121–9.
Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–34.
Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S42–7.
Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86.
Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomised clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882–91.
Aroda VR, Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev. 2011;27:528–42.
Macconell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes. 2012;5:29–41.
Grimm M, Han J, Weaver C, Griffin P, Schulteis CT, Dong H, et al. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the duration trials. Postgrad Med. 2013;125:47–57.
Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–9.
Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care. 2014;37:2500–7.
Yabe D, Seino Y, Fukushima M, Seino S. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:602.
Acknowledgements
This research was funded by the Nanjing Public Health Bureau Project (no. YKK11110), the Science and Technology Support Program of Jiangsu Province (CN) (no. BL2014010), and the China Postdoctoral Science Foundation (2015M581829). All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Disclosures
Feng-fei Li, Lanlan Jiang, Liyuan Fu, Hong-hong Zhu, Peihua Zhou, Danfeng Zhang, Xiao-fei Su, Jin-dan Wu, Lei Ye, and Jian-hua Ma have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of Nanjing First Hospital and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients before they were included in the study.
Data Availability
Datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Li, L. Jiang, L. Fu, H. Zhu contributed equally to this article.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/B947F06059971958.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, Ff., Jiang, L., Fu, L. et al. Exenatide Add-on to Continuous Subcutaneous Insulin Infusion Therapy Reduces Bolus Insulin Doses in Patients with Type 2 Diabetes: A Randomized, Controlled, Open-Label Trial. Diabetes Ther 8, 177–187 (2017). https://doi.org/10.1007/s13300-016-0222-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0222-7