Abstract
Introduction
The objective of this study was to assess basal insulin persistence, associated factors, and economic outcomes for insulin-naïve people with type 2 diabetes mellitus (T2DM) in Japan.
Methods
People aged at least 18 years with T2DM with first claim for basal insulin between May 2006 and April 2013 (index date), no insulin use before index date, and continuous insurance coverage for 6 months before (baseline) and 12 months after index date were selected from the Japan Medical Center Database. On the basis of whether there were at least 30-day gaps in basal insulin treatment, patients were classified as continuers (no gap), interrupters (at least one prescription after gap), and discontinuers (no prescription after gap). A multinomial logistic regression model identified factors associated with persistence. Annual healthcare resource use and costs in the year after initiation were compared between continuers and interrupters and between continuers and discontinuers using propensity score-based inverse probability weighting to adjust for baseline differences.
Results
Of the 827 people included (mean age 50 years, ca. 71% male), 36% continued, 42% interrupted, and 22% discontinued basal insulin therapy in the year after initiation. Having at least one inpatient visit and using fewer classes of non-insulin antihyperglycemic medications during baseline were associated with lower likelihoods of continuing therapy. Relative to interrupters and discontinuers, continuers had lower hospitalization rates [continuers, 12.7%; interrupters, 25.4% (p < 0.001); discontinuers, 28.4% (p < 0.001)] and lower inpatient costs [continuers, ¥132,013; interrupters, ¥225,745 (p = 0.054); discontinuers, ¥320,582 (p = 0.036)], but higher pharmacy costs [continuers, ¥158,403; interrupters, ¥134,301 (p = 0.039); discontinuers, ¥121,593 (p = 0.002)] in the year after insulin initiation. Total healthcare costs were similar for the three cohorts.
Conclusions
Substantial proportions of people with T2DM in Japan interrupt or discontinue basal insulin within the year after initiation, and they have higher rates and costs of hospitalizations than patients who continue with their insulin therapy. Further research is needed to understand reasons behind basal insulin persistence and the implications thereof to help clinicians manage T2DM more effectively.
Funding
Eli Lilly and Company, Boehringer Ingelheim.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM), the most common form of diabetes, affects about 7.6% of adults aged 20–79 years in Japan [1] and accounts for approximately 6% of the national healthcare expenditure [2]. While much of the estimated healthcare cost is attributable to treatment of diabetes itself, a substantial amount of the cost for people with T2DM is associated with the treatment of chronic complications arising as a result of poor glycemic control [2]. Therefore, maintaining adequate glycemic control among people with T2DM is very important for the patients, providers, and healthcare system. The consensus-based guidelines provided by the Japan Diabetes Society (JDS) recommend a stepwise treatment algorithm for effective management of T2DM [3]. According to this algorithm, when lifestyle and diet modifications are inadequate to maintain glycemic control, people with T2DM initiate treatment with an oral hypoglycemic agent (e.g., metformin, sulfonylureas) or an injectable (i.e., insulin or glucagon-like peptide-1 [GLP-1] receptor agonists). Depending on the degree of hyperglycemia, other injectable or oral hypoglycemic agents may subsequently be added to the regimen to achieve adequate glycemic control [3].
Intensive insulin treatment has been shown to be the most effective glucose-lowering therapy; achieving good glycemic control in turn helps prevent the development of micro- and macrovascular complications of diabetes [3,4,5]. Although insulin can help patients achieve their glycemic targets, several studies across multiple countries have reported suboptimal persistence to insulin treatment (i.e., continuous use of insulin) in the real world. For example, in a study of a large commercially insured insulin-naïve population in the USA, Perez-Nieves et al. found that 20% of the patients initiating basal insulin continued treatment in the year after initiation without any interruption [6]. The rates of persistence were even lower in the second year after treatment initiation, with only 46% of those with continuous use in the first year also continuing use in the second year. Similarly, Ascher-Svanum et al. found that only 20% of the people with T2DM who initiated insulin continued treatment beyond the first 90 days after initiation [7]. Other studies have found that the rates of persistence to basal insulin over 1 year [8,9,10,11] or 2 years [12] in various countries range from 18% to 59% among those initiating neutral protamine Hagedorn (NPH) insulin and up to 67% among those using insulin glargine or insulin detemir. Particularly for Japan, in a survey of people with diabetes (both T1DM and T2DM) across several countries, Peyrot et al. found that 43.2% of the respondents in Japan had some level of non-adherence to insulin treatment in the month before the survey [13]. To the best of our knowledge, however, no study to date has evaluated persistence to basal insulin treatment using real-world data in Japan.
Studies have noted that physicians, including those in Japan, are reluctant to initiate insulin treatment for several reasons including physicians’ perception that patients will not be willing to initiate and continue treatment as prescribed [13, 14]. As such, a better understanding of the characteristics of persistent versus non-persistent people in Japan is needed, as having such information can help clinicians manage the care for their patients more effectively. Furthermore, the importance of patients continuing on their prescribed medication has been demonstrated in several studies in the USA which found that discontinuation of insulin therapy is associated with increased use of acute medical care services (e.g., hospitalizations) and costs compared with those continuing the treatment as prescribed [6, 7, 10, 15]. However, the implications of basal insulin persistence among the Japanese population remain to be explored.
Therefore, the goal of the present study was to provide a better understanding of basal insulin persistence, specifically with regards to treatment continuation, interruption, and discontinuation within a year of insulin initiation among people with T2DM in Japan using de-identified administrative health insurance claims data. In addition, the study aimed to assess the factors associated with continuation, interruption, and discontinuation of basal insulin use in the year after treatment initiation and the implications of the different persistence patterns for healthcare resource use and costs during the year after treatment initiation.
Methods
Data and Sample Selection
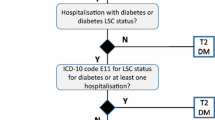
De-identified administrative claims from Japan Medical Data Center, a database containing information on medical and pharmacy services provided between May 1, 2005 and April 30, 2014 for approximately 2 million beneficiaries (persons aged less than 70 years who are employed by middle-to-large size companies in Japan and their dependents), were used for this analysis [16, 17]. The population of interest consisted of beneficiaries with T2DM who had at least one pharmacy claim for basal insulin (insulin glargine, insulin detemir, NPH insulin, insulin degludec) between May 1, 2006 and April 30, 2013. The date of the first pharmacy claim for basal insulin during this time period was defined as the index date, the 6-month period prior to the index date as the baseline period, and the 12-month period following the index date as the follow-up period. Beneficiaries were identified as having T2DM if they met any of the following conditions: (1) at least two diagnoses for T2DM (ICD-10 code E11.x or E14.x) and fewer diagnoses for type 1 diabetes (ICD-10 code E10.x) in the 18-month period comprising the baseline and follow-up periods (“observation period”), OR (2) at least one diagnosis of T2DM during the observation period and at least one prescription for non-insulin antihyperglycemic medication in the 6-month baseline period (an approach consistent with previous research [18, 19]). Further, beneficiaries were required to be at least 18 years old, have no indication of any insulin use (including mixtures and mealtime insulin) in the 6 months prior to and including the index date, and no indication of secondary/other diabetes (ICD-10 codes E08.x, E09.x, E13.x), pregnancy (including gestational diabetes; ICD-10 codes O00.x–O08.x, O10.x–O16.x, O20.x–O29.x, O30.x–O48.x, O60.x, O75.x, O80.x–O92.x, O94.x–O99.x, Z32.1, Z33.x-Z35.x, Z37.x, Z39.x) throughout the baseline and follow-up periods. Beneficiaries were also required to have continuous enrollment in health plans throughout the observation period (to ensure availability of complete pharmacy and medical care information) to be included in the final analytic sample.
This article is based on previously collected data, and does not involve any new studies of human or animal subjects performed by any of the authors.
Basal Insulin Persistence
Availability of the days’ supply for each basal insulin claim was necessary to assess persistence to treatment. However, this information was not directly available from the data; it was derived from the dates of prescriptions using the following approach. First, the median number of days between prescriptions was calculated for the entire sample. Given that the days’ supply may differ as a function of the quantity prescribed, the median days were calculated separately for basal insulin claims with 300 units (median 29 days, 25th percentile 26 days, 90th percentile 56 days) and 600 units or more (median 35 days, 25th percentile 28 days, 90th percentile 63 days) of insulin administered (no dosages that were between 300 and 600 units were observed in the data). Next, for each pair of consecutive claims, the days’ supply were computed as the minimum of the number of days until the patient’s next basal insulin claim and the median number of days between consecutive basal insulin claims observed for the entire sample.
Given the variability in insulin doses, persistence was defined allowing for a grace period (or gap) in available days’ supply of basal insulin. There are two commonly used approaches in the literature to define the maximum allowable gap in treatment: (1) less than 30 days between two consecutive refills [6,7,8], and (2) time between refills that is less than the 90th percentile of the duration between consecutive basal insulin prescription fills for the sample [9, 10, 15]. In this sample, both approaches resulted in similar gap lengths. For the core analyses, the maximum allowable gap in days’ supply was 30 days and the final analytic sample was stratified into three mutually exclusive groups: (1) continuers or persistent users—no gaps of 30 days or more in basal insulin supply during the first 12 months, (2) interrupters—those with at least one basal insulin claim after the first at least 30-day gap in supply of basal insulin during the year after treatment initiation (independent of whether they subsequently discontinued treatment), and (3) discontinuers—those with no basal insulin claims after the first at least 30-day gap in supply of basal insulin during the year after treatment initiation. As a sensitivity analysis, persistence was evaluated allowing for a gap that was less than the 90th percentile of the duration between consecutive basal insulin prescription fills for the sample (see “Sensitivity Analyses Involving Definition of Basal Insulin Persistence” and “Sensitivity Analyses”). During the follow-up period, basal insulin therapy could be the same type as index insulin or other basal insulin or premixed insulin.
In order to better characterize basal insulin use among the interrupters, the number and duration of gaps during the 1-year follow-up period were estimated. Note, it is possible that a patient with at least one interruption subsequently discontinued treatment during the 1-year follow-up period. For the purpose of this analysis, the discontinuation of treatment was considered as another “gap” in treatment and the duration of this “gap” was truncated at the end of the 1-year follow-up period.
Time to first interruption, defined as the time between basal insulin initiation and the day prior to the first at least 30-day gap in the supply of basal insulin, was estimated using Kaplan–Meier analyses. Data for discontinuers were censored at the time of first discontinuation. A similar analysis was conducted to estimate time to discontinuation, with data for interrupters censored at the time of first interruption.
Baseline Characteristics
Differences in demographics (mean age and gender), type of basal insulin used at treatment initiation (analogue vs. human), mode of index basal insulin delivery (i.e., pen or cartridges), Charlson comorbidity index (CCI) [20], and presence of microvascular and macrovascular comorbidities, depression, obesity, other neurological disorders, hypoglycemic events, or dementia during the 6-month baseline period were separately evaluated between continuers and interrupters and between continuers and discontinuers. In addition, measures of medical resource use (likelihood of inpatient and outpatient visits and number of inpatient and outpatient days) as well as prescription drug use (antihyperglycemic medication use: overall and by class of medication; number of unique classes used, proportions using antihypertensives, statins, antidepressants, or antiplatelet agents) during the baseline period were compared across cohorts. Statistical significance of differences was evaluated using Chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Statistical significance was defined as p < 0.05.
Outcomes and Statistical Analyses
Factors Associated with Basal Insulin Persistence
Factors associated with basal insulin interruption and discontinuation were identified using a multinomial logistic regression model (with continuous use as the reference category). All patient characteristics evaluated during the 6-month period prior to basal insulin treatment initiation and at index date (as described above), excluding comorbidities with less than 3% prevalence, were considered as potential factors associated with basal insulin persistence.
Medical Resource Use and Costs Associated with Basal Insulin Persistence
Annual medical resource use stratified by place of service (i.e., inpatient, outpatient) as well as antihyperglycemic medication use in the year after initiation was compared between continuers and interrupters and between continuers and discontinuers. Both all-cause resource use and diabetes-related resource use (defined as claims with ICD-10 codes E11.x or E14.x) were evaluated. In addition, all-cause and diabetes-related medical, pharmacy, and total healthcare costs were compared across the cohorts of interest. Diabetes-related costs included costs associated with diabetes-related medical claims and antihyperglycemic prescription medications.
Differences in underlying characteristics were accounted for using a propensity score-based inverse probability weighting (IPW) method [21]. As with other propensity score-based approaches, the propensity (i.e., likelihood) of being in a given cohort as a function of observed baseline characteristics was estimated using a multinomial logistic regression model. Select baseline and index date characteristics were included as potential independent covariates. Following the computation of propensity scores, each person was attributed a weight defined as the inverse of the propensity score for that person. The weighted baseline characteristics were evaluated to ensure that no important differences remained across cohorts. Statistical comparisons were made between continuers and interrupters and between continuers and discontinuers using weighted t tests for continuous measures and weighted Chi-squared tests for categorical variables. Finally, the medical/prescription drug-related resource use and cost outcomes were compared between continuers and interrupters and between continuers and discontinuers using similar statistical tests as described above.
Medication Use During the First Gap in Treatment
The proportions of interrupters and discontinuers using the various classes of antihyperglycemic medications during the first gap in treatment were evaluated. For discontinuers, this metric was evaluated for the time period between the start of the gap and end of the follow-up period. Prescriptions filled prior to the start of the first gap but with days of supply that overlapped with the gap were included. No comparisons were made within or between the cohorts.
Sensitivity Analyses Involving Definition of Basal Insulin Persistence
Two factors may potentially affect the rates of persistence to basal insulin within this population: (1) the lack of actual days of supply associated with basal insulin claims in the data, and (2) high degree of variability in patient-specific dosing of insulin. Therefore, as a sensitivity analysis, the study estimated the proportions of patients characterized as continuers versus interrupters or discontinuers in the first year after treatment initiation, allowing for gaps of up to 60, 90, and 120 days between available days’ supply for basal insulin. In addition, the study estimated persistence patterns using an alternative definition of persistence that has been used in the literature [9, 10, 15], i.e., allowing for time between refills that is less than the 90th percentile of the duration between consecutive basal insulin prescription fills for the sample, stratified by quantity supplied (300 units and 600 units or more).
Results
Basal Insulin Persistence
A total of 827 people were included in the final analytic sample (Fig. 1). During the first year after treatment initiation, 36% of the people used basal insulin continuously, 42% had at least one gap of 30 days or more between prescriptions, and 22% discontinued therapy after the first gap of at least 30 days (Fig. 2). During the 1-year follow-up period, interrupters had on average 1.9 gaps, with a mean duration of 60.8 days per gap. In regard to timing of interruption, approximately half of the interrupters had their first gap in therapy within the first 90 days following treatment initiation. Based on the Kaplan–Meier analysis that adjusted for censoring, the estimated probability of interruption within 3 months was 23% (Fig. 3). Among the discontinuers, 54% discontinued basal insulin within the first 90 days of treatment initiation. In the Kaplan–Meier analysis, there was a 13% probability that patients discontinued basal insulin within 3 months after initiation (Fig. 3).
Sample selection and resulting patient counts. Non-mixed basal insulins are insulin detemir, insulin glargine, NPH (neutral protamine Hagedorn) insulin, and insulin degludec; T2DM was identified using ICD-10 codes E11.x and E14.x; T1DM was identified using ICD-10 codes E10.x, secondary diabetes as ICD-10 codes E08.x–E09.x, E13.x, and pregnancy as ICD–10 codes O00.x–O08.x, O10.x–O16.x, O20.x–O29.x, O30.x–O48.x, O60.x–O75.x, O80.x–O92.x, O94.x–O99.x, Z32.1, Z33.x–Z35.x, Z37.x, Z39.x
Kaplan–Meier estimates of time to treatment interruption (top) and discontinuation (bottom). When assessing time to interruption, discontinuers were censored at the time of discontinuation. Similarly, when assessing time to discontinuation, interrupters were censored at the time of first interruption
Baseline Characteristics
People using basal insulin continuously were older (51 years vs. 50 years for interrupters and 49 years for discontinuers) (Table 1). In addition, continuers were more likely to have a diagnosis of hypertension or dyslipidemia, used more classes of non-insulin antihyperglycemic medications, and were less likely to use inpatient medical services but more likely to use outpatient medical services in the 6-month baseline period relative to the other two cohorts (Table 1). Within each cohort, 75–77% of people initiated treatment with insulin glargine, 14–18% with insulin detemir, 6–9% with NPH insulin, and 0–1% with insulin degludec. In addition, most (94%) patients, independent of the cohort, used a basal insulin pen at the time of treatment initiation (Table 1).
Factors Associated with Treatment Interruption and Discontinuation
Multivariable models indicated that having at least one hospital visit prior to basal insulin initiation was associated with significantly higher likelihoods of treatment interruption and discontinuation. Use of multiple classes of antihyperglycemic medications was associated with significantly lower likelihoods of treatment interruption and discontinuation. In addition, presence of congestive heart failure was associated with significantly higher likelihood of treatment interruption, while presence of cardiovascular disease (not congestive heart failure) and other neurological disorders was associated with significantly lower likelihood of treatment discontinuation (Table 2).
Follow-up Period Outcomes
Although the three cohorts differed in terms of several of the baseline characteristics evaluated, including age, medical resource use measures, and prescription drug use measures, the inverse probability weighting resulted in cohorts with similar characteristics (Table S1 in the supplementary information). After we accounted for underlying differences, continuers had fewer days hospitalized (2.2 ± 7.8 vs. 4.9 ± 15.6 for interrupters and 6.5 ± 17.6 for discontinuers) in the year after treatment initiation compared with interrupters and discontinuers (Table 3). Consequently, continuers had lower hospital-related costs than interrupters and discontinuers (¥132,013 vs. ¥225,745 for interrupters and ¥320,582 for discontinuers). However, continuers had higher outpatient costs (¥417,046 vs. ¥406,693 for interrupters and ¥314,998 for discontinuers) and pharmacy costs (¥158,403 vs. ¥134,301 for interrupters and ¥121,593 for discontinuers). All comparisons (other than for outpatient costs vs. interrupters) were statistically significant (p < 0.05). As a result, the three cohorts had similar total (i.e., medical plus pharmacy) costs in the year after insulin initiation (Table 3).
In terms of T2DM-related resource use, continuers had fewer days hospitalized than interrupters and discontinuers (2.0 ± 7.3 vs. 4.8 ± 15.2 for interrupters and 6.3 ± 17.3 for discontinuers); whereas, the days with an outpatient visit were similar across the three cohorts. With regards to antihyperglycemic medications, continuers were more likely to use premixed insulin medications, but less likely to use non-insulin injectable medications (i.e., GLP-1 receptor agonists) than the other two cohorts (Table 3). The rates of other antihyperglycemic medication use were similar between continuers and discontinuers, but lower among interrupters. As a result, similar to the all-cause cost results, continuers had similar T2DM-related total and medical costs but had higher pharmacy costs compared to the other two cohorts (Table 3). The finding that the T2DM-related resource use and costs are very similar to the overall resource use and costs suggests that for all the three cohorts, the majority of the estimated all-cause resource use and costs in the year after basal insulin initiation could be attributed to T2DM.
Medication Use During the First Gap
During the first gap, the majority of interrupters and discontinuers used at least one antihyperglycemic medication. Specifically, approximately 19% of the interrupters and 12% of discontinuers used a non-basal (i.e., mealtime) insulin, 1% of the interrupters and 10% of the discontinuers used non-insulin injectable medications (i.e., GLP-1 receptor agonists), and 74% of interrupters and 79% of discontinuers used oral antihyperglycemic medications during the gap (Table S2 in the supplementary material).
Sensitivity Analyses
Results of the sensitivity analyses suggest that the proportions of people characterized as continuers or interrupters/discontinuers during the first year after treatment initiation depend, to a great extent, on the length of allowable gaps in basal insulin use. Allowing for 60-day gaps in basal insulin use would characterize 59% of patients as continuers, 16% as interrupters, and 25% as discontinuers (Table S3 in the supplementary material). Not surprisingly, the persistence rates increase even further when extending the gap length to 90 days (68%) and 120 days (73%). Allowing for gaps shorter than the 90th percentile of days between two consecutive prescription fills during the first year after basal insulin initiation would characterize 31% of the sample as continuers, 49% as interrupters, and 20% as discontinuers (Table S3 in the supplementary material). The resulting proportions are similar to the core analyses, suggesting a high degree of concordance between the two definitions of persistence within this population.
Discussion
In this study, the majority of people with T2DM who initiated basal insulin either used it intermittently (42%) or discontinued (22%) within the first year of treatment initiation, with approximately half doing so within the first 90 days of beginning therapy. The proportions of patients classified as discontinuers remained fairly similar across the different definitions of persistence, suggesting that discontinuation of basal insulin treatment may occur shortly after treatment initiation. Hospital visits and using fewer other antihyperglycemic medications during baseline were associated with significantly higher likelihoods of treatment interruption and discontinuation. During the year after treatment initiation, the interrupters and discontinuers had significantly more days hospitalized, mainly attributable to T2DM, than continuers. Relatedly, continuers had lower hospital-related costs (potentially owing to reduced likelihood of worsening of T2DM and related comorbidities), but higher outpatient and pharmacy costs (potentially associated with continuous medication use and increased interactions with their GPs). Taken together, these components resulted in total medical costs that were similar across the three cohorts. In terms of antihyperglycemic medications, the majority of interrupters and discontinuers used non-basal antihyperglycemic medications during the gap in basal insulin treatment. In fact, during the year after basal insulin initiation, greater proportions of interrupters and discontinuers (compared to continuers) used GLP-1 receptor agonists. These findings suggest that for some patients, an interruption or discontinuation of basal insulin treatment may reflect a change in treatment regimen, e.g., from basal insulin to a GLP-1 receptor agonist to manage their condition. Future research should evaluate the sequence of medications used by T2DM patients in order to manage glycemic control and assess reasons for interruption and discontinuation in basal insulin treatment.
To the best of our knowledge, this is the first study to assess persistence to basal insulin therapy and implications thereof, using real-world health insurance data in Japan. Even so, aspects of our findings are similar to studies conducted in other countries. For example, similar to studies conducted in the USA, we find that the majority of patients interrupt or discontinue basal insulin treatment during the year after initiation [6,7,8]. Similarly, our finding that using multiple antihyperglycemic medications before insulin initiation is associated with a significantly higher likelihood of continuing basal insulin in the year after initiation, while having a hospital visit before insulin initiation is associated with a significantly lower likelihood of insulin continuation is in line with the findings reported in the literature [6, 15]. The association between use of multiple antihyperglycemic medications prior to insulin initiation and greater persistence could likely be explained by better awareness regarding benefits of achieving glycemic control. Another explanation could stem from the fact that persistent users (i.e., continuers) had higher medication use and lower comorbidity rates as well as medical resource use prior to basal insulin initiation, all of which suggest that these patients may generally be more likely to adhere to treatment regimens. Future research should evaluate the association between basal insulin persistence and self-reported factors such as patient education and adherence to other medications.
Several studies have evaluated the economic consequences associated with insulin non-persistence in other countries; however, to the best of our knowledge this is the first study to report similar findings among the Japanese population. Our results take on added importance as we find that continuers had fewer days hospitalized and therefore had lower medical costs during the follow-up period, compared with both interrupters and discontinuers (Table 3). While we did not compare the results between interrupters and discontinuers, the study findings are consistent with results reported by Perez-Nieves et al. using data from the USA [6] and provide important insight regarding the significance of basal insulin persistence. In particular, our findings suggest that any interruption in treatment, even if subsequently reinitiated, is associated with outcomes requiring more acute care, and hence emphasize the importance of patients staying on therapy. However, further research is needed to understand the specific mechanisms behind the association between resource use and costs and persistence to basal insulin, and to better understand the differences within non-persistent patients by comparing the groups of interrupters and discontinuers.
This study has some limitations. First, the analyses relied on accuracy and completeness of administrative claims data for identifying people with T2DM as well as assessing baseline characteristics and outcomes. Second, clinical information about diagnosis or severity of illness (e.g., based on glycemic control) was not captured in the data. Therefore, the relationship between clinical measures such as glycemic control and basal insulin persistence in this study population remains unknown. Relatedly, increased acute care events may also be associated with increased work-loss costs. However, these metrics were not available in the data and therefore do not count towards the estimated burden associated with non-persistence. Third, persistence patterns were assessed on the basis of information regarding dispensed medications as opposed to actual observance of medications taken. In addition, neither days’ supply nor dose of insulin was reported on the claims, and the duration of insulin prescriptions were approximated on the basis of the dates of prescriptions, potentially resulting in inaccurate estimates of actual days’ supply of insulin. Fourth, as noted earlier, both patterns of basal insulin use as well as factors associated with treatment interruption and discontinuation were based on the information captured in the data and do not reflect physician or patient preference on changing course of therapy. Finally, prior studies have noted that the average age of insulin initiation in Japan was 60 years [22, 23]; however, the database does not capture information about people aged 70 years or more. As such, the results are limited to people aged less than 70 years with employer-sponsored insurance in Japan and may not generalize to other populations (e.g., older people, those who are unemployed).
Conclusions
The study findings indicate that the vast majority of people initiating basal insulin in Japan either interrupt or discontinue treatment in the year after initiation (largely within the first 90 days), which is associated with increased medical resource use and costs during the year after initiation. In addition, we find that comorbidity profile, use of other antihyperglycemic medications, and medical resource use prior to treatment initiation are significantly associated with patterns of basal insulin use in the year after initiation. Further research is necessary to better understand the reasons why patients continue, discontinue, or interrupt their insulin therapy. In addition, further studies are needed to understand specific mechanisms behind insulin persistence and its implications to help clinicians manage care for T2DM more effectively.
References
The International Diabetes Federation. Available at: https://www.idf.org/membership/wp/japan. Accessed 1 Sep 2016.
Neville SE, Boye KS, Montgomery WS, et al. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metabolism Res Rev. 2009;25(8):705–19.
The Japan Diabetes Society. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Available at: http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed 1 Sep 2016.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405–12.
Perez-Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32:669–80.
Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5:225–42.
Cooke CE, Lee HY, Tong YP, Haines ST. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin. 2010;26(1):231–8.
Baser O, Tangirala K, Wei W, Xie L. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among US patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;5:497–505.
Wang L, Wei, W, Miao R, Xie L, Baser O. Real-world outcomes of US employees with type 2 diabetes mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMC Open 2013;3: e002348. Available at: http://bmjopen.bmj.com/content/3/4/e002348.full.pdf+html.
Gordon J, Pockett RD, Tetlow AP, McEwan P, Home PD. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64(12):1609–18.
Pscherer S, Chou E, Dippel FW, Rathmann W, Kostev K. Treatment persistence after initiating basal insulin in type 2 diabetes patients: a primary care database analysis. Primary Care Diabetes. 2015;9(5):377–84.
Peyrot M, Barnett AH, Meneghini LF, Schimm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29(5):682–9.
Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes and Needs (DAWN) JAPAN Study. PLoS One. 2012;7(6):e36361. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3375282/.
Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20(1):52–61.
Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413–9.
Japan Medical Data Center. Available at: http://www.jmdc.co.jp/. Accessed 1 Sep 2016.
Perez-Nieves M, Jiang D, Eby E. Incidence, prevalence, and trend analysis of the use of insulin delivery systems in the United States (2005 to 2011). Curr Med Res Opin 2015;31:(5):891–9.
Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36:914–21.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Imbens G. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87(3):706–10.
Kanatsuka A, Kawai K, Hirao K, Japan Diabetes Clinical Data Management Study Group (JDDM), et al. The initiation of insulin therapy in type 2 diabetic patients treated with oral anti-diabetic drugs: an observational study in multiple institutes across Japan (JDDM27). Diabetol Int. 2012;3(3):164–73.
Suzuki S, Ajmera M, Kurosky S, et al. Initiation of basal insulin therapy among patients with diabetes mellitus in Japan: a retrospective analysis in a hospital setting. Presented at the Society of Medical Decision Making (SMDM) 2nd Biennial Asia-Pacific Conference; January 8, 2016. Hong Kong, China.
Acknowledgments
Research funding as well as funding for publication charges is provided by Eli Lilly and Company and Boehringer Ingelheim to Analysis Group, Inc.
All authors made significant contributions to study design, analysis, and interpretation of data, as well as preparing and reviewing the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Previous presentation: Some of the findings from this study were presented at the ISPOR 18th Annual European Congress, Milan, Italy, November 7–11, 2015.
Disclosures
I. Hadjiyianni, D. Cao, and M. Perez-Nieves are employees and stockholders of Eli Lilly and Company. S. Suzuki and D. Chida are employees of Eli Lilly Japan, K.K. U. Desai, J. Ivanova, N. Kirson, and H. Birnbaum are employees of Analysis Group, Inc. C. Enloe was an employee of Analysis Group, Inc. at the time of the study.
Compliance with Ethics Guidelines
This article is based on previously collected data, and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The data used for the study were provided by Japan Medical Data Center (JMDC) to Analysis Group, Inc. The data license agreement does not permit sharing of datasets with people external to the study team. Interested readers may request the data directly from JMDC.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/7037F06030A69805.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hadjiyianni, I., Desai, U., Suzuki, S. et al. Basal Insulin Persistence, Associated Factors, and Outcomes After Treatment Initiation: A Retrospective Database Study Among People with Type 2 Diabetes Mellitus in Japan. Diabetes Ther 8, 149–166 (2017). https://doi.org/10.1007/s13300-016-0215-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0215-6