Abstract
The progressive nature of type 2 diabetes (T2D) often results in the need for initiation and subsequent intensification of insulin treatment to achieve glycemic control. The aim of this review is to examine published clinical evidence that has directly compared two recommended treatment approaches in patients with T2D: (1) a ‘basal plus’ regimen, whereby 1–2 injections of prandial insulin are added to basal insulin; or (2) the use of once- or twice-daily premix insulin analogs, which contain both basal and prandial insulin in a single injection. Broadly, the available evidence suggests that both basal plus and premix regimens are comparable in terms of efficacy and safety when used for insulin initiation in insulin-naïve patients and intensification in patients who have failed on basal insulin; instances of greater glycemic control are observed with premix insulin; however, these are often accompanied by increases in hypoglycemia and/or weight relative to basal plus treatment, and results should be interpreted within the context of total insulin doses used. Relatively low numbers of patients achieved glycemic control when both regimens were used for insulin intensification following failure of basal insulin, suggesting that a full basal–bolus regimen and/or the use of different treatments is clinically indicated in certain patients. In summary, the current review argues that both basal plus and premix insulin regimens are relatively efficacious and safe options for patients with T2D during both insulin initiation in insulin-naïve patients and intensification in patients who have failed on basal insulin. This emphasizes the important role of patient-centered factors in clinical decision-making.
Funding: Novo Nordisk.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is a chronic disease that results in a majority of patients requiring insulin treatment due to the progressive decline in pancreatic β-cell function [1]. Clinical guidelines in many countries, including those of the Australian Diabetes Society (ADS) and International Diabetes Federation (IDF), suggest that insulin can be initiated with either a long-acting basal insulin (e.g., insulin glargine, insulin detemir, insulin degludec) administered once daily (OD), or premix insulin administered OD or twice daily (BID), when lifestyle changes and treatment with glycemic-lowering, oral antidiabetic drugs (OADs) (usually in combination therapy) are no longer sufficient to help the patient achieve recommended glycemic targets [2, 3].
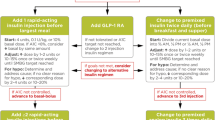
In clinical practice, insulin switching and/or intensification following initiation with basal insulin is commonly required. For example, following 1 year of insulin detemir OD treatment in the Novo Nordisk-sponsored ‘4T’ trial, only 8.1% of patients achieved glycemic control from a baseline glycated hemoglobin (HbA1c) of 8.5% [4]. Switching and/or intensification is often required to reduce postprandial plasma glucose (PPG) excursions; basal-only regimens are unlikely to cover these instances of postprandial hyperglycemia, and closer attention to PPG is usually necessary to achieve tight glycemic targets [5]. Additionally, control of PPG may be an important factor to reduce diabetes-related cardiovascular complications and mortality [6, 7]. For insulin-treated patients with T2D who require intensification, clinical guidelines recommend to: (1) continue with basal insulin OD and add rapid-acting prandial insulin in a ‘stepwise’ manner, up to three-times daily (TID), i.e., a full basal–bolus regimen; or (2) intensify or transfer to premix insulin BID [2, 3].
Premix insulin analogs combine a fixed ratio of rapid-acting and protaminated insulin, which provide both prandial and basal components in a single formulation and are generally administered BID [8]. Common premix insulins include biphasic insulin aspart 30/70 (e.g., NovoMix® 30; Novo Nordisk A/S, Bagsvaerd, Denmark), which contains 30% rapid-acting insulin aspart and 70% intermediate-acting protaminated insulin aspart, and insulin lispro 25/75 (e.g., Humalog® Mix25®; Eli Lilly and Company, Indianapolis, Indiana, United States), which contains 25% insulin lispro injection and 75% protaminated insulin lispro. Newer premix analogs such as insulin degludec/insulin aspart (Ryzodeg®; Novo Nordisk A/S, Bagsvaerd, Denmark) are also gaining popularity in clinical practice. It is thought that up to 40% of patients with T2D currently use premix insulin as part of their treatment, and a number of guidelines are now published for the use of premix insulin when initiating, intensifying, or switching insulin regimens [2, 9, 10].
Compared with basal insulin alone, initiation with premix insulin has shown variable results: greater glycemic control in patients with T2D, but with higher rates of overall hypoglycemia and weight gain shown [4, 11–16]. In the 3-year follow-up of the ‘4T’ trial [NCT00184600], similar median HbA1c levels were achieved, with a lower hypoglycemia rate seen in the basal insulin detemir group [17]. For insulin intensification, a recent meta-analysis reported a small, non-significant difference for HbA1c reduction favoring basal–bolus compared with premix, along with no significant differences in overall hypoglycemia, weight gain, and insulin dose [18]. Broad advantages of premix insulin for patients diagnosed with T2D include convenience, improved PPG control compared with basal insulin alone, a single delivery device, and the ability to intensify treatment up to TID if needed [19]. One current practical guidance suggests that premix should be considered for, among other factors, initiation in patients who have a PPG increment of >3 mmol/L and predictable lifestyle and meal patterns [10]. For intensification, it is recommended that premix regimens should be considered in patients who prefer fewer injections, less frequent self-monitoring of blood glucose, and who may have a diminished ability to inject (e.g., poor manual dexterity) [10].
Recent years have seen an increase in the popularity of the basal plus regimen, i.e., when 1–2 injections of prandial insulin are added prior to the meal(s) associated with the largest PPG excursion [20], which is the first part of stepwise intensification toward the full basal–bolus regimen [3]. An advantage of the basal plus regimen is that it has greater flexibility compared with a premix regimen, but it does require potentially greater complexity as the insulin regimen intensifies over time. For example, it requires the patient to manage two different insulins and injecting devices, and the demands of variable dosing of the short-acting ‘plus’ component according to the carbohydrate content of meals. Nevertheless, the basal plus approach is now being used as a therapeutic alternative to premix BID regimens and is useful to prepare the patient for a full basal–bolus regimen, in those who are comfortable with more frequent injections and the need for increased self-monitoring [10, 11]. The next intensification step after basal failure (stepwise addition of prandial insulin from basal plus to TID), has shown non-inferiority to a full basal–bolus regimen for reducing HbA1c with similar, or lower, rates of hypoglycemia, and similar weight gain [21, 22].
There are a number of existing trials and reviews that have compared premix insulin to a full basal–bolus regimen in patients with T2D [18, 23–26]. However, there is a gap in the literature regarding comparison of the efficacy and safety of basal plus in relation to premix OD and BID regimens in two commonly encountered clinical contexts: (1) insulin initiation in insulin-naïve patients following the failure of lifestyle modification and OADs to achieve target HbA1c; (2) insulin intensification in the context of suboptimal glycemic control, despite adequately titrated basal insulin.
Insulin treatment is conducted in both specialist and primary care settings, with the majority of insulin now being initiated in primary care [24, 27]. Consequently, a review of the available evidence comparing the efficacy and safety of basal plus vs. premix regimens in patients diagnosed with T2D is relevant to the broad audience of healthcare professionals involved in the treatment of such patients.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Clinical Evidence
Insulin Initiation
At the time of writing, two clinical trials (n = 1505) have been published that directly compared basal plus and premix regimens in insulin-naïve patients [9, 28]. Both trials were sponsored by Sanofi and recruited patients with T2D who had HbA1c >7% (>53 mmol/mol) despite at least 3 months of OAD use and lifestyle modification (Table 1). The GALAPAGOS trial [NCT01121835] included a 2-week screening period, in which patients continued on their existing therapy, including diet, exercise, and stable dose of OADs. Following randomization, patients who started with insulin glargine OD or premix OD continued to take metformin, sulfonylureas, glinides, or dipeptidyl peptidase-4 (DPP-4) inhibitors, and any other diabetes treatments, including thiazolidinediones and α-glucosidase inhibitors, were discontinued at this time. Patients who started with or switched to two injections discontinued use of sulfonylureas, glinides, or DPP-4 inhibitors, with metformin therapy remaining unchanged [28]. The second trial by Riddle et al. [9] [NCT00384085] included a 4-week run-in period, in which patients continued existing OADs and replaced insulin secretagogues with an equivalent dose of glimepiride, along with reducing pioglitazone dose to 30 mg/day.
HbA1c
In the GALAPAGOS study, 42.5% of patients in the insulin glargine arm were receiving a basal plus strategy (insulin glargine OD + insulin glulisine OD) by the end of trial (the remaining 57.5% staying on insulin glargine OD), while 63.5% received premix BID (the remaining 36.5% receiving premix OD) [28]. With regard to HbA1c, there was a significantly greater reduction from baseline in HbA1c (P < 0.01) with premix (OD or BID) compared with the insulin glargine OD ± insulin glulisine OD arm. In addition, more patients achieved HbA1c <7% (<53 mmol/mol) (P < 0.01) in the premix (OD or BID) group compared with insulin glargine OD ± insulin glulisine OD [28]. In the Riddle et al. [9] trial, non-inferiority was reported for a basal plus regimen (insulin glargine OD ± insulin glulisine OD) in reducing HbA1c from baseline compared with a premix BID regimen, and there was no difference between the regimens in the proportion of patients achieving HbA1c <7% (<53 mmol/mol).
Insulin Dose and Body Weight
In the GALAPAGOS study, overall insulin dose at end of trial was 0.47 U/kg (36.1 U) in the insulin glargine OD ± insulin glulisine OD arm and 0.61 U/kg (47.2 U) for those treated with a premix (OD or BID) regimen [28]. Riddle et al. [9] reported similar mean weight-adjusted insulin doses in both regimens at end of trial: 0.92 ± 0.47 U/kg/day and 1.04 ± 0.66 U/kg/day in the basal plus and premix regimens, respectively. Unadjusted daily doses at trial endpoint were 93 ± 54.1 U/day and 110 ± 82.3 U/day in the basal plus and premix regimens, respectively [9]. Both trials reported similar weight gain in the respective treatment arms following insulin initiation [9, 28].
Blood Glucose
Fasting plasma glucose (FPG) was 6.0 mmol/L at the end of treatment in the insulin glargine OD ± insulin glulisine OD arm and 6.3 mmol/L in the premix (OD or BID) arm in the GALAPAGOS study [28]. The least squares mean change in FPG from baseline was greater with insulin glargine OD ± insulin glulisine OD when compared with premix (OD or BID) (−0.3 mmol/L; 95% confidence interval [CI]: −0.5; −0.2; P < 0.001) and the mean daily BG reduction was significantly greater with the premix (OD or BID) regimen (P = 0.024) [28]. In the Riddle et al. [9] trial, mean FPG at end of trial (or last observation carried forward) was lower with a basal-plus regimen than with premix BID (6.7 vs. 7.9 mmol/L; P < 0.01).
Safety
The GALAPAGOS study reported a significantly lower incidence of overall symptomatic hypoglycemia with insulin glargine OD ± insulin glulisine OD when compared with premix (OD or BID) (≤3.1 mmol/L: 1.20 vs. 2.93 events/patient-year; P < 0.01, respectively) (≤3.9 mmol/L: 4.85 vs. 8.37 events/patient-year; P < 0.01, respectively) [28]. The significantly lower rate of hypoglycemia with insulin glargine OD ± insulin glulisine OD compared with premix (OD or BID) was also observed for nocturnal symptomatic hypoglycemia (≤3.1 mmol/L: 0.35 vs. 1.03 events/patient-year; P < 0.01, respectively) (≤3.9 mmol/L: 1.14 vs. 2.28 events/patient-year; P < 0.01, respectively) [28]. Similarly, Riddle et al. [9] showed a significantly lower incidence of symptomatic hypoglycemia with a basal plus regimen when compared with a premix regimen (<2.8 mmol/L: 0.8 vs. 1.9 events/patient-year; P < 0.01, respectively) (<3.9 mmol/L: 7.1 vs. 12.2 events/patient-year; P < 0.01, respectively). Severe hypoglycemia was similar in both basal plus and premix regimens (0.1 vs. 0.2 events/patient-year; respectively).
Other adverse events (AEs) or treatment-emergent adverse events (TEAEs) did not differ greatly between treatments in either trial [9, 28]. A similar percentage of patients treated with insulin glargine OD ± insulin glulisine OD or premix (OD or BID) experienced at least one TEAE (34.6% vs. 35.7%) in the GALAPAGOS study; serious TEAEs were reported in 2.6% of the basal plus group and 5.0% of the premix group. One fatal TEAE (pulmonary embolism) occurred in the basal plus group [28]. The Riddle et al. [9] trial observed AEs in 79% and 80% of patients in the premixed and basal plus insulin regimens, respectively, and serious AEs were reported in 10.8% of the premix group and 12.4% of the basal plus group. AEs related to the study drug were not distinguished.
Insulin Intensification
Three published trials (n = 970) have directly compared basal plus and premix regimens in patients with T2D uncontrolled with basal insulin OD plus OADs [29–31] (Table 2). Sponsored by Sanofi, Jin et al. [29] [NCT01212913] had an initial 12-week period whereby insulin was titrated to stabilize insulin doses in both treatment arms, resulting in insulin doses being similar in both arms from week 12 onwards. Sponsored by Eli Lilly, patients in the Tinahones et al. [30] trial [NCT01175824] underwent a 2-week screening period, after which they were randomized to treatment. Patients continued taking metformin and/or pioglitazone throughout, unless changes arising from safety concerns were required. In the LanScape study [NCT00965549], sponsored by Sanofi, patients were randomized only if they had HbA1c >7% (>53 mmol/mol) and a mean self-monitored FPG of <7 mmol/L following an initial 8- or 12-week run-in period of basal insulin optimization [31].
HbA1c
Jin et al. assessed intensification with basal plus (insulin glargine OD + insulin glulisine [OD or BID]) and premix BID in Korean patients with T2D [29]. This study is of interest as there may be differences in the general context of the typically high-carbohydrate diet and known insulin secretory deficits that characterize Asian T2D [32]. Prandial insulin glulisine was administered OD in 50% of patients and BID in the remaining 50%. With regard to HbA1c, similar reductions from baseline were observed in both insulin glargine OD + insulin glulisine (OD or BID) and premix BID regimens, along with the proportion of patients achieving HbA1c <7% (<53 mmol/mol) [29].
Tinahones et al. [30] observed a greater reduction in HbA1c for a premix BID regimen compared with basal plus (insulin glargine OD + insulin lispro OD) (P < 0.05). However, there was no difference between the regimens in the proportion of patients achieving HbA1c <7% (<53 mmol/mol) [30]. A similar pattern was seen in the LanScape study, with no significant differences observed between a basal plus (insulin glargine OD + insulin glulisine OD) and premix BID regimen in reduction in HbA1c and proportion of patients achieving HbA1c <7% (<53 mmol/mol) [31].
Insulin Dose and Body Weight
A lower daily insulin dose in the premix BID arm compared with the basal plus arm was initially observed in the Jin et al. [29] trial, but this was gradually up-titrated, such that end of trial insulin dose was similar in both regimens. Tinahones et al. [30] did not report a significant difference in mean insulin dose at end of trial between basal plus and premix regimens (50.8 vs. 53.1 U, respectively). In the LanScape study, total daily insulin dose increased from baseline to end of trial in both basal plus and premix regimens (+25.5 vs. +35.6 U, respectively) [31]. For all three insulin intensification trials, weight gain was similar in both basal plus and premix BID regimens, with no clinically relevant differences being observed [29–31].
Blood Glucose
Jin et al. [29] reported that the mean increase in FPG was lower in the insulin glargine OD + insulin glulisine (OD or BID) regimen when compared with premix BID (3.1 mg/dL vs. 24.4 mg/dL; P < 0.01, respectively). The seven-point self-measured blood glucose (SMBG) profile change from baseline was significantly lower in the insulin glargine OD + insulin glulisine (OD or BID) arm when compared with premix BID before breakfast (−4.27 mg/dL vs. 16.24 mg/dL; P < 0.01, respectively) and 2 h after lunch (−59.42 mg/dL vs. 32.0 mg/dL; P < 0.01, respectively). Tinahones et al. (2014) reported that change in FPG from baseline was similar in both the basal plus and premix BID regimen (0.75 mmol/L vs. 0.89 mmol/L, respectively), while in a 7-point SMBG curve at end of trial, blood glucose was significantly lower in the basal plus regimen before breakfast (6.26 mmol/L vs. 6.60 mmol/L; P < 0.01) and lower in the premix BID group before lunch (6.82 mmol/L vs. 7.44 mmol/L; P < 0.001) [30]. The LanScape study did not report blood glucose outcomes for the treatment period [31].
Patient-Reported Outcomes
Both the Tinahones et al. trial and LanScape study included patient-reported outcomes following treatment [30, 31]. Tinahones et al. [30] reported no significant difference between regimens for patient-reported treatment satisfaction, along with the perceived emotional and physical effects of treatment. The LanScape study, however, reported significant differences in favor of basal plus for total treatment satisfaction, total insulin satisfaction, present quality of life (QoL), and perceived frequency of hyperglycemia. However, no significant differences between the groups were observed for the perceived frequency of hypoglycemia, change in the average weighted impact of diabetes on QoL, or health status [31].
Safety
Jin et al. reported that, for weeks 0–12, incidence of overall hypoglycemia was higher in the insulin glargine OD + insulin glulisine (OD or BID) arm compared with premix BID (818 events vs. 375 events; P < 0.05, respectively). For weeks 12–24, the rate of overall hypoglycemia was 152 vs. 386 (P = 0.23) events for insulin glargine OD + insulin glulisine (OD or BID) and premix BID regimens, respectively. Statistical differences in the rate of nocturnal hypoglycemia in the insulin glargine OD + insulin glulisine (OD or BID) and premix BID regimens were not observed for weeks 0–12 (86 vs. 61 events, respectively; P = 0.11) and weeks 12–24 (22 vs. 57 events, respectively; P = 0.67), along with severe hypoglycemia: weeks 0–12 (1 vs. 0 events, respectively; P = 0.49) and weeks 12–24 (one event for each; P = 0.74) [29].
Tinahones et al. [30] reported similar rates of overall hypoglycemia in both basal plus and premix BID regimens (≤3.9 mmol/L: 16.5 vs. 13 events/patients-year, respectively), along with nocturnal hypoglycemia (≤3.9 mmol/L: 1.8 vs. 1.5 events/patients-year, respectively). No severe hypoglycemia was observed in either treatment arm. No significant difference between basal plus and premix regimens in overall confirmed hypoglycemia (estimated rate ratio [RR]: 0.84; 95% CI: 0.64;1.11; P = not significant [NS]) was observed in the LanScape study [31]. Nocturnal hypoglycemia was significantly lower in the premix group (RR: 1.57; 95% CI: 1.08;2.29; P < 0.05), and severe hypoglycemia occurred in 13 (7.6%) basal plus patients and in nine (5.5%) premix patients [31].
As with insulin-naïve patients, there were similar numbers of TEAEs between the two intensification regimens. Of the 107 AEs reported in the Jin et al. [29] trial, one AE was judged to be related to insulin glargine OD + insulin glulisine (OD or BID) treatment and four to premix BID treatment (no further details regarding the type of AEs are reported). In Tinahones et al. [30], 2.9% of basal plus patients and 3.8% of premix users experienced AEs considered to be possibly related to study treatment; overall serious TEAEs were reported by 3.3% and 4.7% of patients receiving basal plus and premix insulin, respectively. The proportion of patients experiencing overall TEAEs in the LanScape study was 75.3% in the basal plus and 65.9% in the premix BID insulin arm, with the majority of TEAEs being mild. One participant died in each treatment arm; however, neither death was considered to be due to the study treatment [31].
Discussion
Insulin Initiation in Insulin-Naïve Patients
Broadly, in clinical practice, the selection of suitable therapy following failure of OADs and lifestyle modification can be a difficult decision for many clinicians. Although basal insulin is a common first insulin regimen, there are a subset of patients for whom postprandial rises are significant and for whom clinicians may reasonably consider a basal plus or premix regimen from the outset.
With regard to the reviewed trials, as would be expected, HbA1c reductions were greater in insulin-naïve patients exposed to insulin for the first time [9, 28], compared to those undergoing insulin intensification [29–31]. This is to be expected, as some of the benefit of insulin therapy is likely to have already been derived by the group on insulin as opposed to the insulin-naïve cohort.
For the trials assessing insulin initiation, both premix (OD or BID) and basal plus treatment resulted in a reduction in HbA1c from baseline, in patients who had failed on lifestyle modification and OAD treatment [9, 28]. A higher proportion of patients achieved HbA1c <7% (<53 mmol/mol) with premix insulin in both trials, and the GALAPAGOS study reported a significantly greater reduction in HbA1c in premix (OD or BID) insulin compared with insulin glargine OD ± insulin glulisine OD treatment [28]. However, it is possible that the degree of HbA1c lowering was related to the higher total daily dose of premix insulin in individuals, rather than the type of insulin itself.
Both trials reported significantly greater hypoglycemia and numerically higher, although not statistically significant, weight gain with the use of premix [9, 28]. Indeed, the greatest weight gain following insulin initiation was the 6.9 kg observed in the premix BID arm of Riddle et al. [9], compared with a 5.2 kg increase in the basal plus arm. This greater weight gain is likely to be due to the fact that patients in this trial had the highest starting FPG, HbA1c, and insulin doses, and the trial had the longest duration compared with the other trials reviewed here. No clinically significant differences in TEAEs were observed between treatments in either of the insulin initiation trials [9, 28].
In summary, the two reviewed trials report greater HbA1c reductions with premix regimens, but less favorable hypoglycemia profiles and weight gain compared with basal plus, and it is possible that these differences could be a reflection of the level of insulin dosing rather than the regimen itself (Table 3).
Insulin Intensification from Basal Insulin
For insulin intensification, the three published trials indicate that intensification from basal insulin OD ± OADs with either basal plus or premix BID resulted in similar reductions in HbA1c, along with similar proportions of patients achieving HbA1c <7% (<53 mmol/mol) [29–31].
Tinahones et al. [30] indicated a significant advantage for the premix BID regimen in mean HbA1c reduction in the context of significantly greater weight gain, although it can be argued that the increase of around 0.6 kg evident with premix relative to basal plus is unlikely to be clinically significant. The premix BID arm of Tinahones et al. also reported the highest proportion of patients (34.5%) achieving HbA1c <7% (<53 mmol/mol) compared with the premix BID arm in the other two trials [29–31]. However, in secondary analysis, the proportion of patients achieving HbA1c <7% (<53 mmol/mol) in the premix BID arm was not statistically greater than the basal plus arm of the trial [29–31].
Rates of overall hypoglycemia were similar in both regimens across the three trials [29–31]. However, nocturnal hypoglycemia was significantly higher in the basal plus arm compared with the premix arm of the LanScape study [31], which may be attributable to the evening prandial insulin dose in the basal plus arm being titrated to a tight PPG target when compared with the premix group in which only preprandial blood glucose was targeted. Rates of severe hypoglycemia were also similar in both regimens across the three trials [29–31], with, again, the highest rates being observed in the LanScape study. The relatively high rates of severe hypoglycemia in the LanScape study may be partly attributable to the high incidence of accidental overdose observed in the basal plus arm of this trial [31]. Additionally, a result of note from the Jin et al. trial is the lower hypoglycemia observed in the first 12 weeks of premix BID treatment when compared with basal plus, with similar reductions in HbA1c. For clinicians, this finding could be argued to suggest that premix BID therapy that starts with a lower insulin dose (i.e., 6 U BID)—which is then gradually increased—may have benefits in terms of safety (Table 4).
With regard to patient-reported outcomes, one trial showed greater benefits with basal plus compared with premix in overall satisfaction with treatment, satisfaction with insulin, perceived frequency of hyperglycemia, and overall present QoL [31]. Broadly, this could be argued to partially mirror the previously observed increase in patient satisfaction with basal plus relative to a full basal–bolus regimen previously observed in the Novo Nordisk-sponsored FullSTEP study [NCT01165684] [22], while recognizing that the trial of Tinahones et al. did not observe significant differences in these patient-reported outcomes between the basal plus and premix regimens.
Overall, the three intensification trials reported modest weight gains of 0.5–2.5 kg over 24 weeks [29–31]. As with the insulin initiation trials, no clinically significant differences in TEAEs were observed between treatments [9, 28–31].
When considering the three insulin intensification trials together, it is important to note that the proportion of patients achieving HbA1c <7% (<53 mmol/mol) following intensification with basal plus or premix BID treatment was low (20.6–34.5%) [29–31], compared with the 63.5% observed with a full basal–bolus regimen [33]. As such, within the context of clinical guidelines, it is likely that the majority of the patients included in the current review would eventually need further intensification of their insulin regimen with the addition of further daily injections [3, 34], while recognizing that a reduction in insulin treatment can be achieved if other factors improve (i.e., lifestyle modification) [3, 24]. Further work is required to identify any specific characteristics that can identify potential non-responders to insulin intensification with either basal plus or premix regimens [11], and whether adding a further dose of insulin would significantly improve glycemic control in these patients, compared with the addition of alternative treatments (e.g., glucagon-like peptide-1 receptor agonists [GLP-1RAs], sodium–glucose co-transporter-2 [SGLT2] inhibitors and DPP-4 inhibitors) [3, 35, 36].
Strengths and Weaknesses
Strengths of the reviewed trials included the multinational design of the majority of the trials, which improves the overall generalizability of the results. Furthermore, the majority of intensification trials had either a run-in or selection criteria that ensured that patients had inadequate glycemic control despite good FPG control, suggesting the need to intensify therapy to target PPG. The patient sample also had a relatively long duration of diabetes, which mimics the delay in insulin initiation and intensification often witnessed in real-world clinical practice [37, 38].
With regard to weaknesses, all trials were open-label, and only two trials used a central laboratory measurement for HbA1c. There was also some heterogeneity observed in the patient sample; for instance, the Jin et al. trial recruited Korean patients diagnosed with T2D with a markedly lower body mass index and longer duration of illness compared to the other insulin intensification trials [29–31]. There were also differences in the primary endpoints used and the type and dose of OADs, along with the premix regimens (i.e., both OD and BID regimens being included). The use of premix TID was also not assessed, which has previously shown non-inferiority to a full basal–bolus regimen for glycemic control [25]. Additionally, there were differences observed with regard to the basal plus algorithms employed, with Jin et al. [29] including prandial insulin OD and BID within their basal plus arm and the GALAPAGOS study including prandial insulin OD as part of a larger, basal-only OD arm [28]. All of the trials were sponsored by pharmaceutical companies, although the authors maintained final responsibility for the publications.
Differences in titration algorithms and hypoglycemia definitions were also observed and, therefore, when interpreting the data, it is important to consider that differences in hypoglycemia rates seen across the trials were likely to have been driven not just by insulin type and doses, but also by the study titration strategy and co-administration of insulin secretogogues. It is also likely that hypoglycemia rates will be lower the earlier insulin therapy is commenced (i.e., at a shorter duration of T2D than in the included trials) due to preservation of counter-regulatory responses to glucose lowering. The duration of the included trials was also relatively short, with the longest trial showing a high rate of attrition [9].
For the current review, a measure of statistical heterogeneity and quality assessment would have been of interest, and would have improved the methodological quality. Moreover, combination therapy with newer agents (e.g., GLP-1RAs, SGLT2 inhibitors) was not studied, but is relevant to clinical decision-making when considering treatment options for patients with T2D.
Conclusion
The current review indicates that the similarities between basal plus and premix (OD and BID) regimens when either initiating or intensifying insulin treatment could be argued to be generally greater than the differences, and superiority in one area may be at the cost of increased AEs (e.g., greater HbA1c reduction with increased hypoglycemia). However, it is important to note that hypoglycemia and weight gain arising from the regimens included in the current review may be lower than is commonly feared by patients, and this ‘psychological insulin resistance’ often contributes to a delay in insulin initiation and intensification [37, 39].
When initiating and intensifying insulin treatment, guidelines from bodies such as the ADS and the IDF recommend that clinicians use discretion and a patient-centered approach to treatment [2, 3]. Indeed, when considering that the results of the current review suggest that both basal plus and premix regimens have comparable efficacy and safety in both insulin initiation and intensification contexts, the patient-centered approach becomes of heightened importance within T2D treatment. Patient preference and QoL outcomes should be considered and include elements not assessed in the current review, such as patients’ lifestyle (e.g., hours of work) and dietary habits. For instance, a premix regimen may be more suitable for patients with regular meals and a large, consistent carbohydrate intake. Additionally, a basal plus regimen may be more appropriate for patients with a more varied meal and activity pattern. Moreover, factors such as patients’ health literacy and ability to manage two different insulins in a basal plus regimen vs. one pen in a premix regimen, overall hypoglycemia risk, likely adherence to insulin therapy, and available healthcare resources should be considered [2, 3, 11]. It should also be considered that premixed insulins need to be adequately mixed or gently shaken by patients to ensure safety of administration and dose.
However, while recognizing these factors, the current review suggests that both basal plus and premix insulin regimens remain, and are likely to continue being, relatively efficacious and safe options for patients with T2D.
References
Cnop M, Welsh N, Jonas J-C, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–107.
Gunton JE, Cheung NW, Davis TM, Zoungas S, Colagiuri S. A new blood glucose management algorithm for type 2 diabetes: a position statement of the Australian Diabetes Society. Med J Aust. 2014;201:650–3.
International Diabetes Federation. Global guideline for type 2 diabetes. 2012. http://www.idf.org/global-guideline-type-2-diabetes-2012. Accessed May 1, 2016.
Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–30.
Monnier L, Colette C, Owens DR. Integrating glycaemic variability in the glycaemic disorders of type 2 diabetes: a move towards a unified glucose tetrad concept. Diabetes Metab Res Rev. 2009;25:393–402.
De Vegt F, Dekker J, Ruhe H, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–31.
Yu PC, Bosnyak Z, Ceriello A. The importance of glycated haemoglobin (HbA1c) and postprandial glucose (PPG) control on cardiovascular outcomes in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:1–9.
Garber AJ, Ligthelm R, Christiansen JS, Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab. 2007;9:630–9.
Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal–bolus up to three prandial injections. Diabetes Obes Metab. 2014;16:396–402.
Wu T, Betty B, Downie M, et al. Practical guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015;6:273–87.
Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management—exploring the concept of the basal-plus approach in clinical practice. Diabet Med. 2013;30:276–88.
Ilag LL, Kerr L, Malone JK, Tan MH. Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparison. Clin Ther. 2007;29:1254–70.
Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5.
Pontiroli AE, Miele L, Morabito A. Metabolic control and risk of hypoglycaemia during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2012;14:433–46.
Strojek K, Bebakar WM, Khutsoane DT, et al. Once-daily initiation with biphasic insulin aspart 30 versus insulin glargine in patients with type 2 diabetes inadequately controlled with oral drugs: an open-label, multinational RCT. Curr Med Res Opin. 2009;25:2887–94.
Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes. 2006;114:527–32.
Holman R, Farmer A, Davies MJ, et al. Three year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–47.
Giugliano D, Chiodini P, Maiorino MI, Bellastella G, Esposito K. Intensification of insulin therapy with basal–bolus or premixed insulin regimens in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2016;51:417–28.
Tambascia MA, Nery M, Gross JL, Ermetice MN, de Oliveira CP. Evidence-based clinical use of insulin premixtures. Diabetol Metab Syndr. 2013;5:50.
Lankisch MR, Del Prato S, Dain MP, Mullins P, Owens DR. Use of a basal-plus insulin regimen in persons with type 2 diabetes stratified by age and body mass index: a pooled analysis of four clinical trials. Prim Care Diabetes. 2016;10:51–9.
Davidson M, Raskin P, Tanenberg R, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395–403.
Rodbard HW, Visco VE, Andersen H, Hiort LC, Shu DH. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal–bolus therapy (FullSTEP Study): a randomised, treat-to-target clinical trial. Lancet Diabetes Endocrinol. 2014;2:30–7.
Bellido V, Suarez L, Rodriguez MG, et al. Comparison of basal–bolus and premixed insulin regimens in hospitalized patients with type 2 diabetes. Diabetes Care. 2015;38:2211–6.
Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal–bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care. 2013;36:S212–8.
Jia W, Xiao X, Ji Q, et al. Comparison of thrice-daily premixed insulin (insulin lispro premix) with basal–bolus (insulin glargine once-daily plus thrice-daily prandial insulin lispro) therapy in east Asian patients with type 2 diabetes insufficiently controlled with twice-daily premixed insulin: an open-label, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:254–62.
Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11:45–52.
Blak BT, Smith HT, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with type 2 diabetes in UK primary care. Diabet Med. 2012;29:e191–8.
Aschner P, Sethi B, Gomez-Peralta F, et al. Insulin glargine compared with premixed insulin for management of insulin-naive type 2 diabetes patients uncontrolled on oral antidiabetic drugs: the open-label, randomized GALAPAGOS study. J Diabetes Compl. 2015;29:838–45.
Jin SM, Kim JH, Min KW et al. Basal-prandial versus premixed insulin in patients with type 2 diabetes requiring insulin intensification after basal insulin optimization: a 24-week randomized non-inferiority trial. J Diabetes. 2015;8:405–13.
Tinahones FJ, Gross JL, Onaca A, Cleall S, Rodriguez A. Insulin lispro low mixture twice daily versus basal insulin glargine once daily and prandial insulin lispro once daily in patients with type 2 diabetes requiring insulin intensification: a randomized phase IV trial. Diabetes Obes Metab. 2014;16:963–70.
Vora J, Cohen N, Evans M, Hockey A, Speight J, Whately-Smith C. Intensifying insulin regimen after basal insulin optimization in adults with type 2 diabetes: a 24-week, randomized, open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape). Diabetes Obes Metab. 2015;17:1133–41.
Lee BW, Kang HW, Heo JS, et al. Insulin secretory defect plays a major role in the development of diabetes in patients with distal pancreatectomy. Metabolism. 2006;55:135–41.
Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011;34:510–7.
Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8:58–66.
Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317–32.
Wilding JPH, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62.
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–7.
Davis TME, Davis Cyllene Uwa Edu Au WA, Bruce DG. Glycaemic levels triggering intensification of therapy in type 2 diabetes in the community: the Fremantle Diabetes Study. Med J Aust. 2006;184:325–8.
Brod M, Kongso JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18:23–32.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novo Nordisk. Medical writing and submission support were provided by Liam Gillies, Daniella Pfeifer and Helen Marshall of Watermeadow Medical—an Ashfield company, part of UDG Healthcare PLC, funded by Novo Nordisk A/S. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this review, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
M. Downie has received speaker’s honorarium and partial travel sponsorship to attend meetings from Novo Nordisk and Sanofi. J. Wong independently, and on behalf of institutions with which she is associated, has received research funds, travel grants and speaker/advisory honoraria from various companies including Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk, Merck, AstraZeneca, Bristol-Meyers Squibb, Novartis, Sanofi and Servier. G. Kilov has attended advisory boards or provided peer-to-peer education for Sanofi, Novo, Lilly, Novartis, MSD, Takeda, Astra Zeneca and Abbott.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/B0E6F0606980ACBA.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Downie, M., Kilov, G. & Wong, J. Initiation and Intensification Strategies in Type 2 Diabetes Management: A Comparison of Basal Plus and Premix Regimens. Diabetes Ther 7, 641–657 (2016). https://doi.org/10.1007/s13300-016-0199-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0199-2