Abstract
Introduction
The objective of this study was to assess the effect of sitagliptin on insulin dose in patients with inadequately controlled type 2 diabetes who titrate basal insulin to a target fasting glucose level after initiating sitagliptin.
Methods
This was a multicenter, randomized, double-blind, placebo-controlled, 24-week clinical trial in which treatment with sitagliptin 100 mg/day or placebo was administered concurrently with insulin glargine titration, targeting a fasting glucose of 4.0–5.6 mmol/L (72–100 mg/dL). The trial randomized 660 patients with type 2 diabetes and inadequate glycemic control on insulin, with or without metformin (≥1500 mg/day) or sulfonylurea, for ≥10 weeks. Patients could remain on metformin but not sulfonylurea after randomization.
Results
The increase from baseline in the daily dose of insulin was less in the sitagliptin group (N = 329) compared to placebo (N = 329) (between group difference = −4.7 IU [95% confidence interval [CI] −8.3, −1.2]; p = 0.009). Patients in the sitagliptin group had lower glycated hemoglobin (HbA1c) levels after 24 weeks (between-group difference of −0.4% [95% CI −0.6, −0.3; −4.9 mmol/mol (95% CI −6.6, −3.2)]; p < 0.001), and more patients in the sitagliptin group reached the HbA1c goal of <7.0% (53 mmol/mol), with a between-group difference of 17.3% (95% CI 10.4%, 24.1%; p < 0.001). Fewer patients in the sitagliptin group experienced an adverse event of hypoglycemia (between-group difference = −15.5%, p < 0.001).
Conclusion
Administration of sitagliptin prior to intensive titration of basal insulin glargine reduces the insulin dose requirement while providing superior glycemic control and less hypoglycemia, compared to an insulin-only regimen.
Funding
Merck & Co., Inc., Kenilworth, NJ, USA.
Similar content being viewed by others
Introduction
Patients with type 2 diabetes typically experience progressive deterioration in glycemic control over time, which has been primarily attributed to progressive loss of beta cell function [1–3]. Consequently, most patients will require intensification of therapy to maintain glycemic control by the addition of other antihyperglycemic agents to ongoing treatment. Eventually, therapy with insulin is required by many patients with type 2 diabetes. Typically, basal insulin is used by patients with type 2 diabetes initiating insulin therapy [4]. However, although insulin is the most efficacious glucose-lowering agent currently available, as many as 40–70% of patients treated with insulin fail to achieve glycemic goals [5–8]. This failure may have a number of explanations, including patient and physician factors, such as the occurrence or risk of hypoglycemia, which limit aggressive titration of insulin dose [9]. In addition, while treatment with basal insulin targets fasting and pre-meal blood glucose levels [4], the progressive diminution in insulin secretory capacity in patients with type 2 diabetes can lead to poor post-prandial glycemic control, and as a result, poor overall glycemic control.
Numerous studies have demonstrated improved glycemic control when an oral agent has been added to insulin therapy in patients with type 2 diabetes [10–19]. However, these studies have generally been performed in the context of stable doses of insulin. The addition of thiazolidinediones, metformin, or sulfonylureas to ongoing therapy, in study designs in which the dose of insulin was to remain stable unless reductions were required for hypoglycemia, has been shown to result in reductions in insulin doses relative to the addition of placebo [12, 14–18], presumably related to more down-titration of insulin in the active treatment groups. Similar observations have not been made when dipeptidyl peptidase-4 (DPP-4) inhibitors were added to ongoing therapy with stable doses of insulin [10, 11, 13, 19]. In one study, Vilsbøll et al. [11] reported that the addition of sitagliptin to ongoing therapy with a stable dose of insulin (with or without metformin) provided better glycemic control after 24 weeks relative to a placebo [11]. However, in that study, the incidence of hypoglycemia in the sitagliptin group (16%), while lower than what has been observed in similar studies with other DPP-4 inhibitors [10, 13, 19], was higher than that observed in the placebo group (8%) [11].
The impact of the addition of a DPP-4 inhibitor to treatment of patients who are actively up-titrating basal insulin using a structured treatment algorithm targeting fasting glucose has not been studied. Sitagliptin lowers post-prandial glucose levels by enhancing insulin secretion and by suppressing glucagon production. It was hypothesized that the addition of sitagliptin, compared with a placebo, would result in similar or better glycemic control with a lower dose of insulin (i.e., would be ‘insulin-sparing’) in patients with type 2 diabetes who are actively up-titrating the dose of basal insulin (with or without metformin) to achieve target fasting glucose levels of 4.0–5.6 mmol/L (72–100 mg/dL).
Methods
Study Design
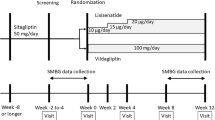
This was a multicenter, randomized, double-blind, placebo-controlled clinical trial (Figure S1) performed between January 16, 2012 and June 07, 2013. The duration of the study was up to 29 weeks, including a 1-week screening period, a 2-week period for sulfonylurea wash-off (for patients who were on sulfonylurea at the screening visit) and/or insulin switch and standardization (for patients who were not on insulin glargine once daily in the evening) (Table S1), a 2-week single-blind placebo run-in period and a 24-week double-blind treatment period, during which the starting insulin dose was titrated based on pre-specified fasting finger-stick glucose measurements starting from Week 2 (Table 1), targeting a fasting glucose of 4.0–5.6 mmol/L (72–100 mg/dL). Patients were randomized (1:1) to sitagliptin 100 mg/day or placebo, and randomization was stratified based on use of metformin and/or a sulfonylurea at the screening visit.
Patients
Patients were eligible for the study if they were 18–80 years of age with type 2 diabetes. Patients who were diagnosed with type 2 diabetes at or after age 40 years were eligible if they had insulin therapy initiated at least 3 years after the diagnosis of diabetes. Patients diagnosed before age 40 years, or patients diagnosed after age 40 who had insulin initiated within 3 years of diagnosis, were also eligible if they had a fasting C-peptide (connecting-peptide) of >0.7 ng/mL. Patients on a stable dose of insulin glargine administered in the evening, with or without metformin (≥1500 mg/day) for ≥10 weeks and with inadequate glycemic control (defined as glycated hemoglobin [HbA1c] ≥7.5% [58.5 mmol/mol] and ≤11.0% [96.7 mmol/mol] at the screening visit) were eligible to begin single-blind placebo run-in at Week −2. Patients on a stable dose of insulin (pre-mixed insulin, or basal insulin other than insulin glargine given in the evening) with or without metformin for ≥10 weeks, with HbA1c ≥7.5% (58.5 mmol/mol) and ≤11.0% (96.7 mmol/mol) at the screening visit were eligible to begin single-blind placebo run-in after a 2-week period to switch and stabilize their insulin dose (to insulin glargine given in the evening). Patients who were on a stable dose of premixed or basal insulin with or without metformin for ≥10 weeks and were also receiving a sulfonylurea, with HbA1c ≥7.5% (58.5 mmol/mol) and ≤10.0% (85.8 mmol/mol) at the screening visit were also eligible to begin the single-blind placebo run-in after a 2-week period to wash-off the sulfonylurea, switch to insulin glargine and stabilize the dose if necessary (i.e., for those who were not on insulin glargine given in the evening). At the start of the single-blind placebo run-in, all patients were required to have FPG ≥7.2 mmol/L (130 mg/dL) and ≤15.0 mmol/L (270 mg/dL).
Patients were excluded from the study if they had type 1 diabetes, a history of ketoacidosis, active liver disease, significant and active cardiovascular disease, malignancy, hematological disorders or hyperthyroidism. Patients were also excluded if they had been treated with a DPP-4 inhibitor, a glucagon-like peptide-1 receptor agonist or a thiazolidinedione within the 12 weeks prior to randomization. Patients were also excluded if they were currently being treated with the daily use of pre-prandial, short-acting or rapid-acting insulin alone, or as part of a basal/bolus insulin regimen. Patients with a history of two or more episodes of hypoglycemia resulting in seizure, coma, loss of consciousness, or with recurrent (≥3 times per week) episodes of hypoglycemia during the 8 weeks preceding randomization were also excluded.
Laboratory exclusion criteria included serum creatinine ≥1.4 mg/dL (males) or ≥1.3 mg/dL (females), an estimated glomerular filtration rate <60 mL/min/1.73 m2 (calculated by modification of diet in renal disease equation), alanine aminotransferase or aspartate aminotransferase >2 times the upper limit of normal, hemoglobin <12 g/dL (male) or <11 g/dL (female), triglycerides >600 mg/dL or thyroid-stimulating hormone outside the normal range.
Study Objectives
The primary objectives for the present study were first to assess the effect of sitagliptin on the change in insulin dose (in IU per day), when compared with a placebo and second, to assess the safety and tolerability of sitagliptin when used in patients who are intensively titrating basal insulin. The primary hypothesis of the study was that after 24 weeks, sitagliptin reduces the dose of insulin relative to a placebo.
Secondary objectives included the assessment of the difference between the effects of sitagliptin and placebo on change from baseline in HbA1c, fasting plasma glucose (FPG), body weight, the percentage of patients who achieve the fasting glucose target of 4.0–5.6 mmol/L (72–100 mg/dL) and the time it took to achieve this target.
Efficacy Endpoints
Efficacy endpoints included daily insulin dose (reported in patients’ self-recorded finger-stick glucose logs), laboratory assessment of HbA1c and FPG, glycemic goal assessment (at or not at goal, defined per protocol as 3 consecutive daily fasting glucose measurements of 4.0–5.6 mmol/L (72–100 mg/dL), obtained from patients’ self-recorded finger-stick glucose logs), and a post hoc analysis of the percentage of patients with HbA1c <7.0% (53 mmol/mol) at Week 24 (or the last visit prior to discontinuation).
Safety Measurements
Safety and tolerability were assessed through the collection and analysis of adverse events (AEs), vital signs, standard laboratory evaluations (including blood chemistry, hematology, and urinalysis), body weight and hypoglycemia assessment logs. All AEs were rated by the study investigators for intensity and relationship to study drug.
Symptomatic hypoglycemia was a pre-specified AE of special interest. Any episode with symptoms consistent with hypoglycemia was reported as an episode of symptomatic hypoglycemia without a requirement for confirmatory blood glucose values and reported as an AE. Episodes with no symptoms, but with a measured blood glucose level of ≤3.9 mmol/L (70 mg/dL), were reported as asymptomatic hypoglycemia; these episodes could be reported as AEs at the discretion of the investigator. Episodes of hypoglycemia that required assistance, either medical or non-medical, were defined as severe hypoglycemia. Episodes with a markedly depressed level of consciousness, loss of consciousness, or seizure were classified as having required medical assistance, whether or not medical assistance was obtained.
Statistical Methods
The population for all efficacy analyses consisted of all randomized patients who received at least one dose of study medication and had at least one measurement of the analysis endpoint at or after baseline. The statistical software used was SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
The primary efficacy endpoint, mean change from baseline in daily insulin dose at Week 24, was compared between the sitagliptin group and the placebo group using a longitudinal data analysis model [20] with a constraint that both groups came from a single population with a common baseline mean. The model adjusted for the patients’ use of metformin at the screening visit (i.e., on metformin, or not on metformin). The model included the following time points: Weeks 0, 2, 4, 6, 8, 10, 12, 15, 18, 21, and 24. Missing outcome data were handled implicitly by the model without the need for imputation. In the analysis model, the daily insulin dose for any given week was defined as the average dose from the three most recent days preceding the index date for that week. The continuous secondary endpoints of mean change from baseline in HbA1c and FPG at week 24 were analyzed using a model analogous to that for the primary endpoint, including the time points of Weeks 0, 2 (for FPG only), 6, 12, 18, and 24. Statistical testing of between-group differences for these secondary endpoints was not pre-specified, but nominal p values (i.e., unadjusted for multiple comparisons) were calculated. The planned sample size of the study was 600 in total, to achieve 90% power (2-sided, α = 0.05), to detect a difference of 6.8% IU of daily insulin dose assuming a standard deviation (SD) of 25 IU, or 9.5 IU assuming a SD of 35 IU. The percentage of patients with HbA1c <7% (53 mmol/mol) at Week 24 (or at the last visit if a patient discontinued before Week 24) were analyzed using the Miettinen and Nurminen method [21] stratified by metformin use at the screening visit. The time to first attainment of fasting glucose target of 4.0–5.6 mmol/L (72–100 mg/dL) was analyzed using the Kaplan–Meier method.
Analyses of safety data included all randomized patients who received at least one dose of study medication. The Miettinen and Nurminen method [21] was used for between-group comparisons of the percentage of patients with safety endpoints. Calculation of a p value for the between-group comparison for symptomatic hypoglycemia was pre-specified. The continuous safety endpoint of body weight was analyzed using the same approach used for the continuous efficacy endpoints.
Statement of Ethics Compliance
The study (Sitagliptin Protocol 260; ClinicalTrials.gov #NCT01462266) was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
A total of 1217 patients were screened, and 557 patients were excluded (Figure S2), most commonly for screen failure (45.3% not meeting the Visit 1 HbA1c criteria or the Visit 1 insulin regimen criterion, and 29.4% for exclusionary laboratory evaluation values). A total of 660 patients were randomized to double-blind treatment (Figure S2) with either sitagliptin 100 mg once daily (330 patients) or matching placebo (330 patients). Two randomized patients who received no study medication, one in each treatment group, were excluded from all analyses. Of the 658 patients who took at least one dose of study medication, 598 (90.6%) completed the trial, of whom 295 were in the sitagliptin group and 303 were in the placebo group.
The baseline mean insulin dose in all treated patients was 36.9 IU/day, mean HbA1c was 8.7% (71.6 mmol/mol), mean FPG was 9.8 mmol/L (176.5 mg/dL), and the mean duration of diabetes was 13.5 years (range 1–33 years). Demographic and anthropometric traits and baseline disease characteristics were generally balanced between the treatment groups (Table 2). Similar proportions of patients in each treatment group had been treated with sulfonylureas, or had been on pre-mixed insulins, prior to randomization (data not shown).
Change from Baseline in Insulin Dose
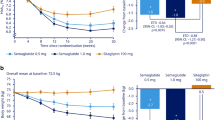
The mean insulin dose increased in both treatment groups over 24 weeks, with most of the increase occurring in the first 15 weeks in both groups (Fig. 1a). In the test of the primary study hypothesis, the change from baseline in daily insulin dose after 24 weeks in the sitagliptin group was significantly smaller than that in the placebo group (p = 0.009; Table 3).
Change from Baseline in Glycemic Parameters
HbA1c
HbA1c levels decreased in both groups (Fig. 1b), with greater reduction from baseline at Week 24 in the sitagliptin group relative to the placebo group (between-group difference −0.4% [−4.9 mmol/mol], p < 0.001; Table 3). After Week 12, the magnitude of the between-group difference was similar throughout the remainder of the trial.
FPG
At Week 24, reductions from baseline in FPG were observed in both groups, with a larger reduction in the sitagliptin group relative to the placebo group (between-group difference −0.6 mmol/L, p = 0.001; Table 3). FPG decreased in both groups through Week 18 with an apparent plateau in both groups from Week 18 through Week 24 (Fig. 1c); a greater decrease in the sitagliptin group was seen from the first post-randomization measurement at Week 2 through the remainder of the trial.
Achievement of Glycemic Goal (HbA1c <7% [53 mmol/mol])
In this post hoc analysis, the percentage of patients with HbA1c <7.0% (53 mmol/mol) at Week 24 (or the last visit prior to discontinuation) was greater in the sitagliptin group compared to the placebo group (Fig. 2). The between-group difference was 17.3% ([95% confidence interval [CI] 10.4%, 24.1%]; p < 0.001).
Achievement of the Fasting Glucose Target; 4.0–5.6 mmol/L (72–100 mg/dL)
The Kaplan–Meier method was used to estimate 25th, 50th, and 75th percentiles of the time to the first attainment of the fasting glucose target. The percentiles were numerically shorter in the sitagliptin group relative to the placebo group; for example, the estimated 50th percentiles for attaining the fasting glucose target were 78 days (95% CI 64, 85) versus 90 days (95% CI 80, 107) for sitagliptin and placebo, respectively (Table 4).
The Kaplan–Meier estimate of the cumulative percentage of patients who had their first attainment of fasting glucose target for each treatment group by Week 24 was slightly higher in the sitagliptin group (77.4%, [95% CI 72.6, 82.2]) relative to the placebo group (74.1% [69.0, 79.2]). The between-group difference in percentages was 3.3% (95% CI −3.7, 10.3).
Safety
Incidences of AEs were similar between the two treatment groups (Table 5). In the sitagliptin group, 64.7% of patients were reported to have any AE vs 69.9% in the placebo group and no notable differences in the incidence of AEs by system organ class were observed. A similar percentage of patients in the sitagliptin group (4.0%) and in the placebo group (3.6%) was reported to have serious AEs (SAEs). No specific SAE term was reported in more than one patient in either treatment group and none of the SAEs were assessed by the investigator as drug related. No patient in the sitagliptin group discontinued study medication due to an SAE while two patients in the placebo group discontinued study medication due to an SAE, one of acute myocardial infarction and the other of cellulitis and sepsis.
The percentage of patients reporting at least one AE of hypoglycemia, either symptomatic or asymptomatic, was lower in the sitagliptin group (28.3%) compared with the placebo group (43.8%), with a between-group difference of −15.5% ([95% CI −22.7, −8.2]; p < 0.001) (Fig. 3). A significantly lower percentage of patients in the sitagliptin group experienced at least one AE of symptomatic hypoglycemia compared with the placebo group (25.2% vs. 36.8%; Fig. 3; p = 0.001); the percentage of patients who reported recurrent symptomatic hypoglycemia (3 or more episodes) was lower in the sitagliptin group (9.4% vs. 19.1%). A lower percentage of patients in the sitagliptin group also reported at least one AE of asymptomatic hypoglycemia compared with the placebo group (9.1% vs. 16.1%; Fig. 3). Most episodes of hypoglycemia in both treatment groups were mild or moderate in intensity, and the percentage of patients with at least one AE of severe hypoglycemia was not meaningfully different between treatment groups (3.0% sitagliptin vs. 4.0% placebo); (Fig. 3).
At Week 24, small increases in body weight were observed in both treatment groups (0.6 kg in the placebo group and 0.3 kg in the sitagliptin group). The 95% CI around the between-group difference of 0.3 kg was −0.8, 0.2.
Discussion
In patients with type 2 diabetes who are not achieving glycemic targets on one or more oral antihyperglycemic agents, current guidelines recommend the addition of a basal insulin [4]. While various algorithms for initiating and intensifying basal insulin therapy have been described and evaluated, they all share a common strategy of dose adjustment based on a targeted fasting blood glucose concentration [5, 7, 8]. This strategy is effective in improving glycemic control but many patients remain above the HbA1c treatment goal of <7.0% (53 mmol/mol) despite aggressive insulin titration [5, 7, 8]. Further insulin titration may be limited by the associated increased incidence of hypoglycemia, which may cause substantial health risks to patients. In a meta-analysis of 67 randomized clinical trials of insulin treatment in type 2 diabetes patients, Pontiroli et al. [22] found significant associations between the incidence of hypoglycemia and both the absolute reduction in HbA1c level and the final achieved HbA1c level. In this context, less stringent glycemic goals are recommended for patients at increased risk for hypoglycemia.
For patients with type 2 diabetes who are treated with insulin, reductions in total insulin dose without sacrificing glycemic control may be of clinical benefit. Higher insulin doses have been associated with an increased risk of hypoglycemia [23], and may also result in greater weight gain [24]. While the use of oral antihyperglycemic agents in combination with insulin would be expected to lower total insulin requirements, the impact of such combinations on these other endpoints is of potential clinical importance.
In the present study, patients titrating basal insulin to a target fasting glucose level of 4.0–5.6 mmol/L (72–100 mg/dL) while being treated with sitagliptin 100 mg/day, compared with a similar group of patients titrating basal insulin while being treated with placebo, had a 20% smaller increase from baseline in insulin dose. Despite this smaller increase in insulin dose (and a lower daily dose of insulin at Week 24), patients in the sitagliptin group had lower HbA1c and FPG levels after 24 weeks, with a higher percentage of patients at HbA1c goal of <7.0% (53 mmol/mol).
As both treatment groups were instructed to follow the same insulin titration algorithm, the study was designed to achieve equipoise between the two treatments with regard to FPG levels. The mean FPG levels appeared to plateau in both treatment groups by Week 18, and >70% of patients in both treatment groups achieved the protocol-specified FPG target of 4.0–5.6 mmol/L (72–100 mg/dL). Despite these observations, which suggest that the study duration and design were adequate to allow for fasting glucose goal attainment, equipoise in FPG between groups was not achieved at Week 24: the mean FPG level in the sitagliptin group was lower than that in the placebo group. Although sitagliptin has been shown to lower FPG in both insulin-treated and non-insulin-treated patients [25–28], this finding was unexpected in this study, as the superimposition of an insulin titration algorithm was expected to compensate for the lack of the effect of sitagliptin in the placebo group. The reason for this lack of equipoise on FPG is not clear. One possible explanation is that the higher incidence of hypoglycemia in the placebo group may have limited the ability to titrate insulin sufficiently or to maintain the insulin dose at the maximally titrated level, although this study was not designed to evaluate the impact of hypoglycemia on titration of insulin. Another possibility is clinical inertia in both treatment groups that resulted in the sitagliptin group having better glycemic control (including a lower mean FPG) due to the additional antihyperglycemic medication. Regarding the differences observed in HbA1c reduction between groups, the greater reduction in post-meal glucose levels in the sitagliptin group may have also contributed to the greater improvement in HbA1c. In keeping with the improved glycemic control achieved in both groups in this study, and possibly due to the titration of insulin, the percentage of patients with an HbA1c <7.0% (53 mmol/mol) at week 24 was more than twice that observed in previous studies in which a DPP-4 inhibitor was added to ongoing stable insulin therapy [10, 11].
Improvement in glycemic control with insulin is typically associated with an increase in the incidence of hypoglycemia. Additionally, previous studies have reported that the incidence of hypoglycemia when an oral agent is combined with insulin is either similar [13, 14, 18–22], or higher [16, 17, 19] compared to the combination of placebo with insulin. Uniquely, in the current study, greater improvement in glycemic control with sitagliptin added on to insulin occurred along with a significantly lower incidence of symptomatic hypoglycemia relative to placebo (25.2% vs. 36.8%), representing a relative reduction of 32% for the sitagliptin group compared to the placebo group.
The lower incidence of hypoglycemia in the sitagliptin group in the current study may be a consequence of the lower dose of insulin used in this group throughout the duration of the trial. Other potential explanations for the lower incidence of hypoglycemia in the sitagliptin group, such as improved counter-regulation in response to hypoglycemia, cannot be excluded, although altered counter-regulation has not been observed with DPP-4 inhibitor treatment. However, it is of interest that the levels of the incretin hormone glucose-dependent-insulinotropic polypeptide (GIP) have been demonstrated to augment glucagon secretion in the setting of hypoglycemia [29–31]. Investigation of the role of stabilization of active GIP by sitagliptin in altering the risk of hypoglycemia will be of interest.
The incidences of hypoglycemia in both the sitagliptin and placebo groups were higher in this study compared to a previous study of sitagliptin added onto stable-dose insulin treatment [11]. However, a cross-study comparison is not appropriate because of the differences in the management of insulin doses between the two studies. In the previous study, sitagliptin was added to ongoing insulin treatment and the insulin dose was kept stable (except for down-titration for hypoglycemia, doses were maintained within 10% of the baseline dose) for the duration of the study [22].
Despite an increase from baseline in the dose of insulin in the sitagliptin group, a minimal increase in weight occurred in this group. The mean change from baseline in body weight of 0.3 kg at Week 24 observed in this study was within the range of the mean change from baseline in body weight observed at Week 24 in studies where a DPP-4 inhibitor was added to stable doses of ongoing insulin therapy [10, 11, 13, 19] despite a greater daily dose of insulin and reduction in HbA1c in this study than in the previous studies.
The results of the current study are consistent with observations made in two recent open-label studies in which sitagliptin was combined with insulin [32, 33]. An open-label, randomized, active comparator controlled, 24-week trial in Korea [32] examined the efficacy and safety of adding sitagliptin to ongoing insulin therapy compared to up-titration of insulin; patients were allowed to remain on stable doses of other antihyperglycemic agents, including sulfonylureas. Compared with up-titration of insulin alone, sitagliptin treatment added to ongoing insulin therapy resulted in a greater decrease in HbA1c and a lower incidence of symptomatic hypoglycemia, even though there was a reduction in the insulin dose. Despite differences in study design, and differing definitions of hypoglycemia, the overall results from our study are consistent with the results from the study in Korean patients [32]. Similarly, an open-label, randomized study of sitagliptin- and metformin-treated patients initiating a twice-daily biphasic insulin while continuing or discontinuing sitagliptin revealed significantly greater reductions in HbA1c and a trend towards lower rates of hypoglycemia, in patients continuing sitagliptin therapy [33].
The limitations of this study include the fact that the study may not have been of sufficient duration to observe the full effect of insulin titration, as insulin doses had not plateaued by Week 24. Additionally, the full impact of the intervention on asymptomatic hypoglycemia and nocturnal hypoglycemia is difficult to assess in the absence of continuous glucose monitoring. Despite these limitations, the results from this study will be useful to guide the treatment of patients who are on basal insulin and wish to titrate their insulin doses based on fasting glucose values.
Conclusions
The ability to achieve glycemic treatment targets with insulin may be limited by the occurrence of hypoglycemia during insulin titration. As demonstrated in this study, the concurrent administration of sitagliptin with a treat-to-target insulin titration regimen reduced the insulin dose requirement while providing superior glycemic efficacy and a reduced incidence of hypoglycemia compared to the treat-to-target insulin-only titration regimen. This study is the first to reflect clinical practice more accurately as to how the combination of a DPP-4 inhibitor and basal insulin is typically administered: a stable dose of the DPP-4 inhibitor and a titration of the basal insulin to fasting glucose target. The data suggest that the addition of the DPP-4 inhibitor sitagliptin may allow for more efficient and safer titration of basal insulin, thus bringing more patients to glycemic target with less risk of hypoglycemia.
References
Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94.
Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the US. Diabetes. 2002;51:2170–8.
Festa A, Williams K, D’Agostino R Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the insulin resistance atherosclerosis study. Diabetes. 2006;55:1114–20.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–8.
Klein R, Klein BE, Moss SE, Cruickshanks KJ. The medical management of hyperglycemia over a 10-year period in people with diabetes. Diabetes Care. 1996;19:744–50.
Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, Tulokas T, Hulme S, Hardy K, McNulty S, Hanninen J, Levanen H, Lahdenpera S, Lehtonen R, Ryysy L. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–51.
Meyer C, Boron A, Plummer E, Voltchenok M, Vedda R. Glulisine versus human regular insulin in combination with glargine in noncritically ill hospitalized patients with type 2 diabetes: a randomized double-blind study. Diabetes Care. 2010;33:2496–501.
Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513–23.
Vilsbøll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77.
Aviles-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182–8.
Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–55.
Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, De Rosa N, D’Onofrio F. Metformin for obese, insulin-treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. Eur J Clin Pharmacol. 1993;44:107–12.
Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–32.
Riddle MC, Schneider J. Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Glimepiride Combination Group. Diabetes Care. 1998;21:1052–7.
Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251–7.
Schwartz S, Raskin P, Fonseca V, Graveline JF. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. N Engl J Med. 1998;338:861–6.
Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, Woerle HJ. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81.
Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Indian J Stat. 2000;62:134–48.
Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26.
Pontiroli AE, Miele L, Morabito A. Metabolic control and risk of hypoglycaemia during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2012;14:433–46.
Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care. 2011;34:1723–8.
Shaefer CF, Reid TS, Dailey G, Mabrey ME, Vlajnic A, Stuhr A, Stewart J, Zhou P. Weight change in patients with type 2 diabetes starting basal insulin therapy: correlates and impact on outcomes. Postgrad Med. 2014;126:93–105.
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45.
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–68.
Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–43.
Farngren J, Persson M, Schweizer A, Foley JE, Ahren B. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2014;16(9):812–8.
Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–9.
Christensen MB, Calanna S, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E418–E26.
Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, Jang HC, Shin H, Walford GA, Lim S. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795–802.
Linjawi S, Sothiratnam R, Sari R, Andersen H, Hiort LC, Rao P. The study of once- and twice-daily biphasic insulin as part 30 (BIAsp 30) with sitagliptin, and twice-daily BIAsp 30 without sitagliptin, in patients with type 2 diabetes uncontrolled on sitagliptin and metformin—The Sit2Mix trial. Prim Care Diabetes. 2015. doi:10.1016/j.pcd.2014.11.001
Acknowledgments
This study and the associated article processing charges were funded by Merck & Co., Inc., Kenilworth, NJ, USA. Editorial assistance was provided by Edward O’Neill, of Merck & Co., Inc., Kenilworth, NJ, USA. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Some of the data reported in this manuscript were previously presented at a World Congress of the International Diabetes Federation (Shankar R, Mathieu C, Lorber D, Umpierrez G, Wu F, Xu L, Golm GT, Latham M, Engel SS, and Katzeff HL. Sitagliptin improved glycemic control with less hypoglycemia and a lower insulin dose in patients who intensify basal insulin therapy. International Diabetes Federation—22nd World Diabetes Congress 2013).
Conflict of interest
R. Ravi Shankar is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Fan Wu is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Lei Xu is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Gregory T. Golm is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Melanie Latham is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Keith D. Kaufman is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Samuel S. Engel is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company. Chantal Mathieu serves or has served on the advisory panel for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, Bristol-Myers Squibb, AstraZeneca, Pfizer, Johnson and Johnson, Boehringer Ingelheim, Hanmi and Mannkind; KU Leuven has received research support from Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Roche, Abbott and Novartis. C. M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Astra Zeneca and Novartis. Daniel Lorber owns stock in Biodel and has served as a paid lecturer for, served as a scientific advisor or consultant to, and received research support from Merck, Novo Nordisk, Janssen, Mannkind, Eli Lilly, Glaxo Smith Kline, Sanofi. Guillermo Umpierrez has received research support to Emory University from Sanofi, Boehringer Ingelheim, Merck, and Novo Nordisk and has participated in advisory panels for Boehringer Ingelheim, Merck, Novo Nordisk, and Sanofi and Regeneron.
Author contributions
Chantal Mathieu, R. Ravi Shankar, Daniel Lorber, Guillermo Umpierrez, Fan Wu, Lei Xu, Gregory T. Golm, Melanie Latham, Keith D. Kaufman, Samuel S. Engel, are responsible for the work described in this paper. Chantal Mathieu was involved with analysis and interpretation of data, and drafting, revising/reviewing the manuscript for important intellectual content. R. Ravi Shankar was involved with study conception and design, interpretation of data, drafting and revising/reviewing the manuscript for important intellectual content. Daniel Lorber was involved with study conception and design, acquisition and interpretation of data, and revising/reviewing the manuscript for important intellectual content. Guillermo Umpierrez was involved with interpretation of study data, and revising/reviewing the manuscript for important intellectual content. Fan Wu was involved with study conception and design, acquisition, statistical analysis and interpretation of data, and revising/reviewing the manuscript for important intellectual content. Lei Xu was involved with statistical analysis and interpretation of data, and revising/reviewing the manuscript for important intellectual content. Gregory T. Golm was involved with study conception and design, statistical analysis and interpretation of data, and revising/reviewing the manuscript for important intellectual content. Melanie Latham was involved with study conception and design, interpretation of data, and drafting the manuscript. K. D. K. was involved with study conception and design, interpretation of data, and revising/reviewing the manuscript for important intellectual content. Samuel S. Engel was involved with study conception and design and interpretation of data, drafting the manuscript and revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Compliance with ethics guidelines
The study (Sitagliptin Protocol 260; ClinicalTrials.gov #NCT01462266) was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov #NCT01462266.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mathieu, C., Shankar, R.R., Lorber, D. et al. A Randomized Clinical Trial to Evaluate the Efficacy and Safety of Co-Administration of Sitagliptin with Intensively Titrated Insulin Glargine. Diabetes Ther 6, 127–142 (2015). https://doi.org/10.1007/s13300-015-0105-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-015-0105-3