Abstract
Introduction
When insulin treatment is started in patients with type 2 diabetes mellitus (T2DM), there are many regimens that control serum glucose levels to a normal range. Basal-bolus insulin therapy is one of the most effective treatments for improving glycemic control to prevent the progression of diabetic microvascular complications. This study was conducted to determine whether step-up insulin treatment with premixed insulin aspart-30/70 (BIAsp 30) or lispro-50/50 (Mix50) in Japanese patients with type 2 diabetes mellitus could achieve better glycemic control.
Methods
In this open label study, 72 insulin-naïve patients with poorly controlled T2DM (HbA1c ≥8.4%), who had been taking oral antidiabetic drugs for at least 12 months, were randomized to receive BIAsp 30 or Mix50 therapy. Patients started treatment of a pre-dinner injection of each type of insulin (Step 1). At 16 ± 2 weeks, a pre-breakfast injection of each type of insulin was added if HbA1c exceeded 7.4% (step 2). If patients had still not achieved HbA1c <7.4% after an additional 16 ± 2 weeks, a pre-lunch insulin injection was added (step 3). Hypoglycemic episodes were also recorded.
Results
The cumulative percentages of subjects who achieved HbA1c <7.4% were 36.1% (13/36) for both Mix50 and BIAsp 30 in step 1, 62.9% (23/36) for BIAsp 30 and 52.8% (19/36) for Mix50 in step 2, and 66.7% (24/36) in BIAsp 30 and 72.2% (26/36) in Mix50 in step 3. The achievement rates of HbA1c <7.4% were not statistically different between the two groups. A total of ten hypoglycemic episodes occurred in this study. However, there were no severe hypoglycemic episodes. All cases recovered by taking glucose and drinking juice.
Conclusion
Mix50 step-up treatment has a clinical effect in achieving good glycemic control equal to that of BIAsp 30 treatment.
Similar content being viewed by others
Introduction
When insulin treatment is started in patients with type 2 diabetes mellitus (T2DM), there are many regimens that control serum glucose levels to a normal range. Basal-bolus insulin therapy is one of the most effective treatments for improving glycemic control to prevent the progression of diabetic microvascular complications [1]. This is because this regimen can stimulate the secretion of insulin from Islets of Langerhans at meal times. However, basal-bolus regimen requires four daily injections [2]. There are other insulin treatments with fewer daily injections which can achieve good glycemic control similar to that achieved with basal-bolus treatment. This is usually preferred by patients with T2DM, as a smaller number of daily injections can maintain their quality of life and compliance [3]. In addition, research by United Kingdom Prospective Diabetes Study (UKPDS) strongly suggests that intensive treatment prevented diabetic microangiopathy complications independent of insulin usage [4]; it is important to maintain good glycemic control levels regardless of multiple or single insulin injections. Recent studies demonstrated that twice-daily treatments with biphasic insulin aspart 70/30 (BIAsp 30) [5, 6] and premixed insulin lispro-50/50 (Mix50) [3] could have equal effects on glycemic control and convey better quality of life than basal-bolus therapy in insulin-naïve patients. In addition, basal insulin (glargine) plus oral treatment showed effects equivalent to basal-bolus treatment [7]. Although these regimens are not always effective for all patients with (T2DM), a therapy with fewer insulin injections might be favored by physicians and patients.
The first 1-2-3 study was performed in the United States [8]. It demonstrated the efficacy of step-up treatment using premixed insulin BIAsp 30, and the practicality of once-daily injection of BIAsp 30 was shown. In addition, twice-daily injection treatment of BIAsp 30, which is used widely [9], was shown to have beneficial effects on glycemic control in a study by Valensi et al. [9]. Subsequently, a similar 1-2-3 study performed in Japan demonstrated that BIAsp 30 step-up therapy was a safe, simple therapy that could achieve better glycemic control [10]. Overall, BIAsp 30 step-up therapy has demonstrated efficacy as a continuing insulin treatment to control glycemic levels regardless of ethnicity.
On the other hand, Mix50 containing 50% lispro and 50% neutral protamine lispro (NPL) is widely used as a twice-daily insulin regimen [11]. The regimen of premixed insulin Mix50 administered three times daily before meals can maintain good glucose control compared with twice-daily injection of humulin 30/70 insulin treatment [12], or with basal plus oral antidiabetic drug (OAD) treatment in insulin-naïve patients with T2DM [13]. However, the beneficial effects of step-up therapy using Mix50 have not been clarified. Therefore, the present study was conducted to determine whether Japanese patients with type 2 diabetes mellitus could achieve better glycemic control with step-up insulin treatment with premixed insulin Mix50 than with BIAsp 30.
Methods
Subjects
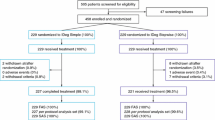
Seventy-two insulin-naïve outpatients with poorly controlled T2DM (glycated hemoglobin A1c [HbA1c] ≥8.4%) aged over 20 years were enrolled. They had been taking OADs for at least 12 months and were randomized to receive BIAsp 30 or Mix50 therapy. Patients with a history of stroke or a cardiovascular event were excluded. Concomitant treatment was stable and maintained unchanged as much as possible throughout the study period. This study was performed at four hospitals (Tochigi). The study protocol was approved by the institutional review board of Dokkyo Medical University Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. All HbA1c data are shown in National Glycohemoglobin Standardization Program (NGSP) values.
Study Design
Patients were randomized by envelope method to two groups: BIAsp 30 or Mix50 group. An HbA1c target of 7.4% was set to minimize the attrition rate and avoid rapid glycemic control improvement, which may have occurred as a result of patients with diabetic complications being included in this study (this HbA1c value corresponded to 7.0% in Japan Diabetes Society [JDS] values). Patients started one injection of 6 units of each type of insulin 15 min before dinner. Patients were instructed not to titrate insulin units by patient themselves to ensure an accurate evaluation. Insulin was decreased by 2 units in the event of morning fasting plasma glucose (FPG) <80 mg/dl and increased insulin amounts 2 units in case of morning FPG >150 mg/dl at every visit (step 1). At 16 weeks, a pre-breakfast injection of 6 units of each type of insulin was added if HbA1c exceeded 7.4%. Insulin was decreased by 2 units in the event of pre-lunch FPG <80 mg/dl and increased by 2 units in the event of re-lunch FPG >150 mg/dl at every visit (step 2). Any oral insulin secreting drugs used during step 1 were discontinued before the patient entered step 2. If patients had still not achieved HbA1c <7.4% after an additional 16 weeks, a pre-lunch insulin injection of 6 units was added. Insulin was decreased by 2 units in the event of pre-dinner FPG <80 mg/dl and increased by 2 units in the event of pre-dinner FPG >150 mg/dl at every visit (step 3). The attending physicians determined the insulin dosage every 4 weeks to achieve HbA1c <7.4% and to avoid hypoglycemic episodes. HbA1c levels, fasting blood glucose, insulin dosage, body weight, body mass index (BMI), and hypoglycemic episodes were investigated at every visit. Dietary education on diabetes was performed within 3 months before starting insulin treatment. HbA1c levels were determined using high-performance liquid chromatography (HPLC: Hi-AUTOA1c, HA8150, Arkray Inc., Kyoto, Japan); all HbA1c data are shown in NGSP values.
Statistical Analysis
All data were analyzed with JMP7® Japanese version analytic software (SAS Japan, Tokyo, Japan). The results are presented as the mean ± standard deviation (SD). To compare the two groups we carried out unpaired t test or the Mann–Whitney U test for continuous variables and the χ2 test or Fisher test for qualitative variables. Two-tailed P values less than 0.05 were considered significant.
Results
Seventy-two patients with T2DM were enrolled in this study. Patient baseline characteristics are shown in Table 1. No significant differences in any parameters at baseline were seen between BIAsp 30 and Mix50 groups (Table 1). Sixty-four patients completed this study, and eight patients (four in BIAsp 30 and four in Mix50 group) refused to the increasing number of insulin injections required to proceed from step 2 to step 3.
Glycemic Control
The cumulative percentage of subjects who achieved HbA1c <7.4% was 36.1% (13/36) for both Mix50 and BIAsp 30 in step 1. The values were 63.9% (23/36) for BIAsp 30 and 52.8% (19/36) for Mix50 in step 2, and 75% (24/32) for BIAsp 30 and 81.3% (26/32 for Mix50 in step 3 (Fig. 1). The cumulative achievement rates of HbA1c were not statistically different between the two groups. Next, among all included patients, 36.1% (13/36) in step 1, 43.5% (10/23) in step 2, and 11.1% (1/9) in step 3 achieved the target HbA1c <7.4% in the BIAsp 30 group, while 36.1% (13/36) in step 1, 26.1% (6/23) in the step 2, and 53.9% (7/13) in step 3 achieved the target HbA1c <7.4% in the Mix50 group.
The HbA1c levels of 9.9 ± 1.7% at the baseline of the study significantly decreased to 7.8 ± 1.0% after 16 weeks (completion of step-1), 7.8 ± 1.0% after 32 weeks (completion of step-2), and 8.2 ± 0.9% after 48 weeks (completion of step-3) in the BIAsp 30 group. In the Mix50n group, HbA1c levels of 9.6 ± 1.6% at the baseline of the study were significantly decreased to 7.8 ± 0.9% after 16 weeks (completion of step-1), 7.6 ± 0.9% after 32 weeks (completion of step-2), and 7.7 ± 0.9% after 48 weeks (completion of step-3). There was no significant difference in HbA1c or FPG between the two groups at any observation point (Table 2).
The HbA1c levels of patients who achieved target HbA1c <7.4% significantly decreased in each step (Table 3); however, the HbA1c levels of patients who did not achieve target HbA1c <7.4% were difficult to reduce regardless of the number of insulin injections and the insulin dosage. In addition, the fasting blood glucose levels of uncontrolled patients did not decrease from step 1 to step 2 in either group.
Insulin Dosage
The daily BIAsp 30 dosages for the patients who achieved target HbA1c <7.4% in step 1 were 0.12 U/kg at the start, 0.18 U/kg at the completion of step 1, and in step 2 were 0.14 U/kg at the start and 0.40 U/kg at the completion of step 2, and in step 3 were 0.11 U/kg at the start and 0.64 U/kg at the completion of step 3. In addition, the daily BIAsp 30 doses for the uncontrolled patients were 0.11 U/kg at the start and 0.63 U/kg at the completion of step 3. The daily Mix50 dosages for the patients who achieved target HbA1c <7.4% in step 1 were 0.11 U/kg at the start, 0.15 U/kg at the completion of step 1, and in step 2 were 0.10 U/kg at the start, 0.32 U/kg at the completion of step 2, and in step 3 were 0.12 U/kg at the start, 0.56 U/kg at the completion of the step 3. In addition, the daily Mix50 dosages for the uncontrolled patients were 0.09 U/kg at the start and 0.56 U/kg at the completion of step 3 (Table 3). The insulin doses between the two groups were not significantly different in each step.
Body Mass Index
No significant change was seen in BMI in either group of patients who achieved target HbA1c in step 1. BMI increased slightly but significantly from 22.5 to 23.4 kg/m2 in BIAsp 30 patients who achieved target HbA1c in step 2. Similarly, the BMI increased slightly but significantly from 22.7 to 23.5 kg/m2 in Mix50 patients who achieved target HbA1c in step 3. The BMI of uncontrolled patients also increased significantly in both groups (Table 3). Moreover, the basal BMI of uncontrolled patients was significantly higher than that of patients who achieved target HbA1c (29.9 ± 8.0 [n = 8] vs. 22.9 ± 2.7 [n = 24] kg/m2 in BIAsp 30 group [P < 0.05], 28.5 ± 6.1 [n = 6] vs. 23.4 ± 2.7 [n = 26] kg/m2 in Mix50 group, respectively [P < 0.05]).
Safety
A total of 10 hypoglycemic episodes occurred in 6 subjects (3 in BIAsp 30 group, 3 in Mix50 group). The details of hypoglycemic episodes (BMI, HbA1c, step, treatment group, OADs, symptom and time when hypoglycemic episode occurred, and achieved step) are as follows. The first case: 20.7, 7.6%, step-1, BIAsp 30, sulfonylurea (SU) and metoformin, tachycardia and sweating, before breakfast, step-1; the second case (2 times): 24.0, 7.8% and 7.5%, step-2, BIAsp 30, metoformin, sweating, 2 times before breakfast, step-2; the third case (2 times): 25.7, 9.2% and 8.8%, step-2 and step-3, BIAsp 30, α-glucosidase inhibitor, headache and tachycardia, before breakfast and before dinner, uncontrolled; the fourth case: 26.0, 8.2%, step-1, Mix50, SU and thiazolidine, sweating, before breakfast, step-2; the fifth case (2 times): 22.5, 8.1% and 7.5%, step-2 and step-3, Mix50, metoformin, sweating, before breakfast and before lunch, step-3; the sixth case (2 times): 37.8, 9.0, and 8.6%, step-2 and step-3, Mix50, α-glucosidase and thiazolidine, sweating and trembling, before breakfast (step-2) and before dinner (step-3), uncontrolled. All cases recovered by taking glucose or drinking juice. However, there were no serious hypoglycemic episodes during the study. In addition, there were no adverse drug reactions related to the BIAsp 30 or Mix50 insulin injections.
Discussion
Graber et al. [8] showed the usefulness of step-up therapy with BIAsp 30 in a 1-2-3 study performed in USA. They indicated that the cumulative rates of patients achieving the target HbA1c ≤6.5% and 7.0% were 21% and 41% with once-daily injection, 52% and 70% with twice-daily injection, and 60% and 77% with thrice-daily injection. Yoshioka et al. [10] demonstrated the usefulness of step-up therapy with BIAsp 30 in Japanese T2DM patients. They showed that the cumulative rates of achievement of HbA1c <6.5% and <7.0% were 5.1% and 21.2% with once-daily injection, 21.2% and 39.4% with twice-daily injections, and 28.3% and 48.5% with thrice-daily injections (including that patients dropped out). Recently, Hosoi et al. [14] also demonstrated the efficacy of step-up therapy with BIAsp 30 in Japanese patients with T2DM. They showed that the cumulative rates of achievement of HbA1c <7.0% were 10.3% with once-daily injection, 41.3% with twice-daily injections, and 51.4% with thrice-daily injections.
Overall, the effectiveness of step-up treatment of BIAsp 30 was recognized regardless of ethnicity. The findings of the present study seem to be better in terms of the cumulative ratio of patients who achieved target HbA1c levels than the previous Japanese study about the treatment with BIAsp 30. A probable explanation for the differences between the results presented here and previous ones is the differences of study protocol with regard to permission for OADs and target HbA1c levels. These findings clearly showed the usefulness of step-up treatment by BIAsp 30, by which 36.1% (13/36) of patients in step 1, 43.5% (10/23) in step 2, and 11.1% (1/9) in step 3 achieved target HbA1c <7.4%. Moreover, the present study demonstrated that insulin step-up treatment with Mix50 showed an effect on glycemic control equal to that with BIAsp 30. Although more increasing number should be needed, the ratio of patients achieving target HbA1c in thrice-daily injection of Mix50 (7/13: 53.8%) was better than that with BIAsp 30 (1/9: 11.1%) (P = 0.04).
There are some merits to initiating insulin treatment by step-up regimen using biphasic insulin. One is that step-up treatment is a simple method because only one insulin device is required, and only once-daily injection before dinner is introduced at the start. Therefore, it is easily acceptable for not only physicians, but also patients as a routine clinical treatment. Previous 1-2-3 studies demonstrated the usefulness of once-daily injection of BIAsp 30 [8, 10]. Moreover, a small dose of BIAsp 30 once a day before dinner in combination with OADs was shown to be effective for glycemic control [15, 16]. On the other hand, there have been no reports about once-daily injection of Mix50 before dinner. Our findings indicated that a high rate (36.1%) of patients achieved the target HbA1c in not only the BIAsp 30 group but also the Mix50 group. In addition, the insulin dosage of patients who achieved the target HbA1c with only once-daily injection was relatively small (0.18 U/kg in the BIAsp 30 group and 0.15 U/kg in the Mix50 group at the completion of step 1). The probable explanation for the effect of once-daily injection of Mix50 before dinner is that for most Japanese the main meal of the day is dinner, which has a high glycemic index since it includes foods such as rice, as Roach et al. [17] demonstrated that the greater proportion of rapid-acting insulin analog was more effective for carbohydrate-rich meals.
The prevalence of diabetes with obesity is increasing worldwide. In general, it is difficult to achieve target glycemic control in the treatment of obese diabetic patients because of high insulin resistance [18]. When insulin is initiated in these patients, higher insulin dosage is required and must be increased [19]. The results of the present study show that the basal BMI of uncontrolled patients who failed to achieve target HbA1c <7.4% regardless of thrice-daily injection of BIAsp 30 or Mix50 was significantly higher than that of patients who achieved target HbA1c. During the study, the BMI of uncontrolled patients increased significantly in both groups. However, the rate of increase of BMI was similar to that of a previous study [20]. In addition, their fasting blood glucose levels did not decrease with the step 2 treatment, suggesting that their insulin resistance might be high, although plasma c-peptide was not assessed in this study. Lifestyle modifications with diet and exercise are the most essential for the management of obese diabetic patients, and combination of OADs to decrease insulin resistance or of basal insulin, such as glargine to decrease the fasting blood glucose and to minimize weight gain compared with that with rapid or premixed insulin, should be considered when insulin therapy is required for the treatment of obese, diabetic patient [21].
There were several limitations in the present study. One of the limitations was that the diurnal plasma glucose measurement was not performed in the outpatient setting and, therefore, the effect of insulin treatment on diurnal plasma glucose change was not fully assessed. Second, the target of HbA1c <7.4% was slightly higher than HbA1c <6.5% that the JDS recommends as good glycemic control to prevent microvascular complications [22]. Although a target HbA1c <7.4% was set to minimize the number of drop-out patients in this study, further studies with tighter and more intensive glycemic control are needed to elucidate the effects of step-up treatment of BIAsp 30 and Mix50 insulin. Third, dipeptidyl peptidase-4 (DPP-4) inhibitor, a novel anti-diabetic agent, was not used in this study. This is because the addition of DPP-4 inhibitor to insulin treatment in patient with diabetes was not permitted in the health care services in Japan during this study period. However, some clinical studies show that adding DPP-4 inhibitors to insulin therapy improves glycemic control without increasing hypoglycemia or weight gain [23, 24], and co-treatment with DPP-4 inhibitors and insulin is permitted in the current clinical diabetic treatment in Japan. Therefore, increased effectiveness of insulin step-up treatment could be observed in patients with T2DM with DPP-4 inhibitors in terms of achievement of glycemic control and without weight gain.
In conclusion, the superiority of step-up treatment with Mix50 insulin was not demonstrated here. However, step-up treatment with Mix50 insulin appears to have a clinical effect in achieving good glycemic control equal to that of treatment with BIAsp 30 in insulin naive patient with T2DM with poor glycemic control. This suggests that step-up treatment by biphasic insulin is a useful regimen to initiate insulin therapy.
References
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17.
Ligthelm RJ, Mouritzen U, Lyggaard H, et al. Biphasic insulin aspart given thrice daily is as efficacious as a basal-bolus insulin regimen with four daily injections: a randomized open-label parallel group four months comparison in patients with type 2 diabetes. Exp Clin Endcrinol Diabetes. 2006;114:511–9.
Masuda H, Sakamoto M, Irie J, Kitaoka A, Shiono K, Inoue G, Atsuda K, Yamada S. Copparison of twice-daily injections of biphasic insulin lispro and basal-bolus therapy: glycaemic control and quality-of-life of insulin-naïve type 2 diabetic patients. Diabetes Obes Metab. 2008;10:1261–5.
United Kingdom Prospective Diabetes (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Hirao K, Arai K, Yamauchi M, Takagi H, Kobayashi M. Six-month multicentric, open-label, randomized trial of twice-daily injections of biphasic insulin aspart 30 versus multiple daily injections of insulin aspart in Japanese type 2 diabetic patients (JDDM11). Diabetes Res Clin Pract. 2008;79:171–6.
Miyashita Y, Nishimura R, Nemoto M, Matsudaira T, Kurata H, Yokota T, Yokota K, Tojo K, Utsunomiya K, Tajima N. Prospective randomized study for optimal insulin therapy in type 2 diabetic patients with secondary failure. Cardiovasc Diabetol. 2008;7:16–24.
Bretzel RG, Nuber U, Landgraf W, Owens D, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomized controlled trial. Lancet. 2008;371:1073–84.
Garber AJ, Wahlen T, Wahl T, Bressler P, Braceras R, Allen E, Jain R. Attainment of glycaemic goals in type 2 diabetes with once-, twice, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8:58–66.
Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, Shah S, Shestakova M, Wenying Y. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMixR 30) in routine care: safety and effectiveness in patients with type 2 diabetes in the IMPROVETM observational study. Int J Clin Pract. 2009;63:522–31.
Yoshioka N, Kurihara Y, Manda N, Komori K, Kato M, Kijima H, Wada N, Yanagisawa K, Aoki S, Ono Y, Koike T. Atep-up therapy with biphasic insulin aspart-70/30-Sapporo 1-2-3 study. Diabetes Res Clin Pract. 2009;85:47–52.
Roach P, Trautmann M, Arora V, Sun B, Anderson JH Jr. Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro mix25 and insulin lispro mix50. Mix50 Study Group. Clin Ther. 1999;21:523–34.
Schernthaner G, Kopp HP, Ristic S, Muzyka B, Peter L, Mitteregger G. Metabolic control in patients with type 2 diabetes using Humalog Mix50 injected three times daily: crossover comp with human insulin 30/70. Horm Metab Res. 2004;36:188–93.
Robbins DC, Beisswnger PJ, Ceriello A, Goldberg RB, Moses RG, Pagkalos EM, Milicevic Z, Jones CA, Sarwat S, Tan MH. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2007;29:2349–64.
Hosoi Y, Ohtani K, Shimizu H, Kashima K, Sato N, Akiyama H, Mori M. Attainment of glycaemic goals by step-up therapy with biphasic insulin aspart-70/30 in Japanese type 2 diabetic patients. Endcrine J. 2011;58:131–5.
Lund SS, Tamow L, Frandsen M, Nielsen BB, Hansen BV, Pedersen O, Parving HH, Naag AA. Combining insulin with metformin or an insulin secretagogue in non-obese patients with type 2 diabetes: 12 month, randomized, double blind trial. BMJ. 2009;339:b4324.
Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, both biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complicat. 2003;17:307–13.
Roach P, Arova V, Campaigne BN, Mattoo V, Rangwala SH. Humalog Mix50 before carbohydrate-rich meals in type 2 diabetes mellitus. Diabetes Obes Metab. 2003;5:311–6.
Dorchy H. What glycemic control can be achieved in young diabetics without residual secretion of endogenous insulin? What is the frequency of severe hypoglycemia and subclinical complication? Arch Pediatr. 1994;1:970–81 (French).
Albu J, Raja-Khan N. The management of the obese diabetic patient. Prim Care. 2003;30:465–91.
Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;65S:S23–7.
Davies M, Khunti K. Insulin management in overweight or obese type 2 diabetes patients: the role of insulin glargine. Diabetes Obese Metab. 2008;10:42–9.
Kobayashi M, Yamazaki K, Hirao K, Oishi M, Kanatsuka A, Yamauchi M, Takagi H, Kawai K. The status of diabetes control and antidiabetic drug therapy in Japan-A cross-sectional survey of 17,000 patients with diabetic mellitus (JDDM 1). Diabetes Res Clin Pract. 2006;73:198–204.
Kothny W, Foley J, Kozlovsky P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–7.
Yki-Jarvien H, Rosenstock J, Duran-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, Woerle HJ. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. Nozomi Domeki and Mihoko Matsumura have contributed to the manuscript in writing, data collection and analysis, and Tsuyoshi Monden and Yuki Nakatani have contributed to data collection, Yoshimasa Aso has contributed in editing assistance. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Nozomi Domeki, Mihoko Matsumura, Tsuyoshi Monden, Yuki Nakatani, and Yoshimasa Aso declare they have no conflict of interest.
Compliance with ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Domeki, N., Matsumura, M., Monden, T. et al. A Randomized Trial of Step-up Treatment with Premixed Insulin Lispro-50/50 vs. Aspart-70/30 in Patients with Type 2 Diabetes Mellitus. Diabetes Ther 5, 403–413 (2014). https://doi.org/10.1007/s13300-014-0078-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-014-0078-7