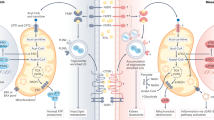
Abstract
Although diabetes is mainly diagnosed based on elevated glucose levels, dyslipidemia is also observed in these patients. Chronic kidney disease (CKD), a frequent occurrence in patients with diabetes, is associated with major abnormalities in circulating lipoproteins and renal lipid metabolism. At baseline, most renal epithelial cells rely on fatty acids as their energy source. CKD, including that which occurs in diabetes, is characterized by tubule epithelial lipid accumulation. Whether this is due to increased uptake or greater local fatty acid synthesis is unknown. We have recently shown that CKD also leads to decreased fatty acid oxidation, which might be an additional mechanism leading to lipid accumulation. Defective fatty acid utilization causes energy depletion resulting in increased apoptosis and dedifferentiation, which in turn contributes to fibrosis and CKD progression. Enhanced fatty acid oxidation in the kidney induced by fenofibrate, a peroxisomal proliferator-activated receptor (PPAR)-α agonist, showed benefit in mouse models of CKD. Fenofibrate treatment also reduced albuminuria in patients with diabetes in multiple clinical trials. Taken together, these findings suggest that further understanding of lipid metabolism in diabetic kidney disease may lead to novel therapeutic approaches.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282.
Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15(6):805–12. This paper provides a thorough review of fatty acid metabolism in the heart and highlights diabetic or obese conditions, alterations in lipid metabolism and possible treatments to alleviate lipid-related pathology.
Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1(1):8–15.
Jiang T et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280(37):32317–25.
Wang W et al. Deletion of scavenger receptor A protects mice from progressive nephropathy independent of lipid control during diet-induced hyperlipidemia. Kidney Int. 2012;81(10):1002–14. This study highlights the important role of a transmembrane receptor in hyperlipidemic kidney and tubular cell injury.
Weinberg JM. Lipotoxicity. Kidney Int. 2006;70(9):1560–6.
Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. 1936;12(1):83–98.
Chajek T, Stein O, Stein Y. Pre- and post-natal development of lipoprotein lipase and hepatic triglyceride hydrolase activity in rat tissues. Atherosclerosis. 1977;26:549–61.
Goldberg IJ et al. Localization of lipoprotein lipase mRNA in selected rat tissues. J Lipid Res. 1989;30(10):1569–77.
Coburn CT et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–9.
Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294(6):F1315–22.
Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19(4):393–402.
Ginsberg HN. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45 Suppl 3:S27–30.
Haemmerle G et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–7.
Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989;320(16):1060–8.
Taskinen MR et al. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol. 2011;31(9):2144–50.
Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290(2):F262–72.
Chauhan V, Vaid M. Dyslipidemia in chronic kidney disease: managing a high-risk combination. Postgrad Med. 2009;121(6):54–61.
Arici M et al. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant. 2002;17(10):1751–7.
Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol. 2002;283(4):F640–7.
Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2(2):e45.
Ruggiero C et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am J Physiol Renal Physiol. 2014;306(8):F896–906.
Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277(21):18919–27.
Wang Z et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54(8):2328–35.
Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68(6):2608–20.
Kang HM et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. This novel study provides evidence for the first time that it is not necessarily the increase in fatty acid levels but its defective oxidation that may play a key role in kidney fibrosis.
Ledo N et al. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol. 2015;26(3):692–714.
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–40.
Muoio DM et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–77. This study provides key evidence for the lipid overload theory in skeletal muscle in contrast to the lipotoxicity theory.
Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973;248(10):3426–32.
Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16(8):757–60.
Vickers AE. Characterization of hepatic mitochondrial injury induced by fatty acid oxidation inhibitors. Toxicol Pathol. 2009;37(1):78–88.
Son NH et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120(10):3443–54.
Liu L et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284(52):36312–23.
Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15(5):595–605. This paper discusses mitochondrial lipid overload and its potential role in insulin sensitivity and emphasizes the advances of this hypothesis.
Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61.
Wang Z et al. Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab. 2000;279(3):E539–45.
Nieth H, Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966;209(5029):1244–5.
Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomark Biochem Indic Expo Response Susceptibility Chem. 2005;10(1):S10–23.
Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):279–86.
Stadler K et al. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45(6):866–74.
Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442(3):453–64. This study highlights that reactive lipids are not only deleterious, but they play major roles in signaling pathways as well due to their reactive, electrophile nature.
Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci. 2002;27(10):489–92.
Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009; 2(90 re7).
Morton J et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care. 2012;35(11):2201–6.
Anonymous. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986.
Baigent C et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92.
Portilla D, Mandel LJ, Bar-Sagi D, Millington DS. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992;262(3 Pt 2):F354–60.
Davis TM et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54(2):280–90.
Ting RD et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study. Diabetes Care. 2012;35(2):218–25. The studies described in this paper collectively summarize the advantages and potential disadvantages of fenofibrate therapy in diabetic kidney disease patients.
Kostapanos MS, Florentin M, Elisaf MS. Fenofibrate and the kidney: an overview. Eur J Clin Investig. 2013;43(5):522–31.
Acknowledgments
Research in the Stadler and Susztak laboratories are supported by DiaComp Pilot and Feasibility grants (14GHSU1393, through Georgia Regents University/NIH) and the Pennington Foundation (K.St), NIH (K.Su) DK076077, DK087635 and HL45095, HL073029, and DK095684 (IJG).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Krisztian Stadler, Ira J. Goldberg, and Katalin Susztak declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Microvascular Complications—Nephropathy
Rights and permissions
About this article
Cite this article
Stadler, K., Goldberg, I.J. & Susztak, K. The Evolving Understanding of the Contribution of Lipid Metabolism to Diabetic Kidney Disease. Curr Diab Rep 15, 40 (2015). https://doi.org/10.1007/s11892-015-0611-8
Published:
DOI: https://doi.org/10.1007/s11892-015-0611-8