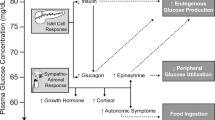
Abstract
Small doses of glucagon given subcutaneously in the research setting by an automated system prevent most cases of hypoglycemia in persons with diabetes. However, glucagon is very unstable and cannot be kept in a portable pump. Glucagon rapidly forms amyloid fibrils, even within the first day after reconstitution. Aggregation eventually leads to insoluble gels, which occlude pump catheters. Fibrillation occurs rapidly at acid pH, but is absent or minimal at alkaline pH values of ~10. Glucagon also degrades over time; this problem is greater at alkaline pH. Several studies suggest that its primary degradative pathway is deamidation, which results in a conversion of asparagine to aspartic acid. A cell-based assay for glucagon bioactivity that assesses glucagon receptor (GluR) activation can screen promising glucagon formulations. However, mammalian hepatocytes are usually problematic as they can lose GluR expression during culture. Assays for cyclic AMP (cAMP) or its downstream effector, protein kinase A (PKA), in engineered cell systems, are more reliable and suitable for inexpensive, high-throughput assessment of bioactivity.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kimball CP, Murlin JR. Aqueous extracts of pancreas: some precipitation reactions of insulin. J Biol Chem. 1923;66:337.
Staub A, Behrens OK. The glucagon content of crystalline insulin preparations. J Clin Invest. 1954;33:1629–33.
• Pedersen JS. The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol. 2010;4:1357–67. An excellent review of the nature of spontaneous glucagon aggregation into amyloid fibrils..
Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2006;355:501–23.
Onoue S, Ohshima K, Debari K, Koh K, Shioda S, Iwasa S, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21:1274–83.
Teoh CL, Griffin MD, Howlett GJ. Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein Cell. 2011;2:116–27.
• Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–32. Very interesting article demonstrating that packing of hormones into amyloid is the natural way that hormones are stored in secretory granules prior to their secretion..
El-Khatib FH, Jiang J, Damiano ER. A feasibility study of bihormonal closed-loop blood glucose control using dual subcutaneous infusion of insulin and glucagon in ambulatory diabetic swine. J Diabetes Sci Technol. 2009;3:789–803.
Ward W, Engle J, Duman H, Bergstrom C, Kim S, Federiuk I. The benefit of subcutaneous glucagon during closed-loop glycemic control in rats with type 1 diabetes. IEEE Sensors J. 2008;8:88–96.
El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2:27ra27.
Castle JR, Engle JM. El Youssef J, Massoud RG, Yuen KC, Kagan R, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–7.
• Ward WK, Massoud RG, Szybala CJ, Engle JM, El Youssef J, Carroll JM, et al. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol. 2010;4:1311–21. An article that demonstrates the near absence of glucagon fibrillation at pH 10..
Jackson M, Stonex T, Caputo N, El Youssef J, Branigan D, Castle JR, et al. Development of a stable formulation of liquid glucagon for use in a bihormonal pump. Presented at 2012 Scientific Session, American Diabetes Association, Philadelphia, PA; 2012.
Robinson NE, Robinson AB. Molecular clocks. Proc Natl Acad Sci U S A. 2001;98:944–9.
Joshi AB, Sawai M, Kearney WR, Kirsch LE. Studies on the mechanism of aspartic acid cleavage and glutamine deamidation in the acidic degradation of glucagon. J Pharm Sci. 2005;94:1912–27.
Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27:544–75.
Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4:1332–7.
Steiner SS, Hauser R, Li M, Feldstein R, Pohl, R. Stabilized glucagon solutions. US Patent Application # 12/891,240.
Chabenne JR, DiMarchi MA, Gelfanov VM, DiMarchi RD. Optimization of the native glucagon sequence for medicinal purposes. J Diabetes Sci Technol. 2010;4:1322–31.
DiMarchi MA, Smiley DL, DiMarchi M, Chabenne JR, Day J. Glucagon analogs exhibiting enhanced solubility and stability. US Patent Application # 12/999,284.
Prestrelski S, Fang WJ, Carpenter JF, Kinzell J. Glucagon formulations for the treatment of hypoglycemia. US Patent Application # 13/186,275.
Jezek J. Stable aqueous systems comprising proteins. US Patent Application 11/994,408.
Verma AK, da Silva JH, Kuhl DR. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother. 2004;38:544–9.
Ward WK, Castle JR, Branigan D, Massoud RG. El Youssef J. Discomfort from an alkaline formulation delivered subcutaneously in humans: albumin at pH 7 vs pH 10. Clin Drug Investig. 2012;32:433–8.
Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels. 2002;8:179–88.
Almholt K, Tullin S, Skyggebjerg O, Scudder K, Thastrup O, Terry R. Changes in intracellular cAMP reported by a Redistribution assay using a cAMP-dependent protein kinase-green fluorescent protein chimera. Cell Signal. 2004;16:907–20.
Jiang Y, Cypess AM, Muse ED, Wu CR, Unson CG, Merrifield RB, et al. Glucagon receptor activates extracellular signal-regulated protein kinase 1/2 via cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2001;98:10102–7.
Pilar Lopez M, Gomez-Lechon MJ, Castell JV. Role of glucose, insulin, and glucagon in glycogen mobilization in human hepatocytes. Diabetes. 1991;40:263–8.
Gomez-Lechon MJ, Ponsoda X, Castell JV. A microassay for measuring glycogen in 96-well-cultured cells. Anal Biochem. 1996;236:296–301.
Cypess AM, Unson CG, Wu CR, Sakmar TP. Two cytoplasmic loops of the glucagon receptor are required to elevate cAMP or intracellular calcium. J Biol Chem. 1999;274:19455–64.
Cascieri MA, Koch GE, Ber E, Sadowski SJ, Louizides D, de Laszlo SE, et al. Characterization of a novel, non-peptidyl antagonist of the human glucagon receptor. J Biol Chem. 1999;274:8694–7.
Parker JC, McPherson RK, Andrews KM, Levy CB, Dubins JS, Chin JE, et al. Effects of skyrin, a receptor-selective glucagon antagonist, in rat and human hepatocytes. Diabetes. 2000;49:2079–86.
Ikegami T, Cypess AM, Bouscarel B. Modulation of glucagon receptor expression and response in transfected human embryonic kidney cells. Am J Physiol Cell Physiol. 2001;281:C1396–402.
Acknowledgments
This work was financially supported in part by grants from Juvenile Diabetes Research Foundation (#17-2012-15), the Legacy Good Samaritan Foundation, and by NIH (NCRR,ORIP) grant # P51OD011092.
Disclosure
Conflicts of interest: MA Jackson: none N Caputo: has received grant support from the Juvenile Diabetes Research Foundation; and Novo Nordisk provided glucagon for research purposes as an in kind donation; JR Castle: has received grant support from the Juvenile Diabetes Research Foundation; and is on the Speaker's Bureau for Amylin; LL David: none; CT Roberts Jr: has received grant support from the Juvenile Diabetes Research Foundation; WK Ward: has received grant support from the Juvenile Diabetes Research Foundation; has received support for travel to meetings for the study or otherwise from the Juvenile Diabetes Research Foundation; Novo Nordisk provided glucagon for research purposes as an in kind donation; is on the scientific advisory board of Xerix Pharmaceuticals; some of the authors filed patents regarding methods of chemically stabilizing glucagon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jackson, M.A., Caputo, N., Castle, J.R. et al. Stable Liquid Glucagon Formulations for Rescue Treatment and Bi-Hormonal Closed-Loop Pancreas. Curr Diab Rep 12, 705–710 (2012). https://doi.org/10.1007/s11892-012-0320-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0320-5