Abstract
Purpose
Activity of acromegaly is gauged by levels of GH and IGF-1 and epidemiological studies demonstrate that their normalization reduces acromegaly’s excess mortality rate. However, few data are available linking IGF-1 levels to features of the disease that may relate to cardiovascular (CV) risk. Therefore, we tested the hypothesis that serum IGF-1 levels relative to the upper normal limit relate to insulin sensitivity, serum CV risk markers and body composition in acromegaly.
Methods
In this prospective, cross-sectional study conducted at a pituitary tumor referral center we studied 138 adult acromegaly patients, newly diagnosed and previously treated surgically, with fasting and post-oral glucose levels of endocrine and CV risk markers and body composition assessed by DXA.
Results
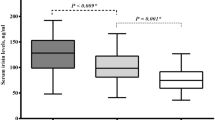
Active acromegaly is associated with lower insulin sensitivity, body fat and CRP levels than acromegaly in remission. %ULN IGF-1 strongly predicts insulin sensitivity, better than GH and this persists after adjustment for body fat and lean tissue mass. %ULN IGF-1 also relates inversely to CRP levels and fat mass, positively to lean tissue and skeletal muscle estimated (SME) by DXA, but not to blood pressure, lipids, BMI or waist circumference. Gender interacts with the IGF-1-lean tissue mass relationship.
Conclusions
Active acromegaly presents a unique combination of features associated with CV risk, reduced insulin sensitivity yet lower body fat and lower levels of some serum CV risk markers, a pattern that is reversed in remission. %ULN IGF-1 levels strongly predict these features. Given the known increased CV risk of active acromegaly, these findings suggest that of these factors insulin resistance is most strongly related to disease activity and potentially to the increased CV risk of active acromegaly.




Similar content being viewed by others
References
Holdaway IM, Rajasoorya RC, Gamble GD (2004) Factors influencing mortality in acromegaly. J Clin Endocrinol Metab 89(2):667–674
Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly [see comments]. J Clin Endocrinol Metab 83(10):3419–3426
Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S, Gomez JM, Halperin I, Lucas-Morante T, Moreno B, Obiols G, de Pablos P, Paramo C, Pico A, Torres E, Varela C, Vazquez JA, Zamora J, Albareda M, Gilabert M (2004) Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 151(4):439–446
Lombardi G, Galdiero M, Auriemma RS, Pivonello R, Colao A (2006) Acromegaly and the cardiovascular system. Neuroendocrinology 83(3–4):211–217
Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK (1994) Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf). 41(1):95–102
Puder JJ, Nilavar S, Post KD, Freda PU (2005) Relationship between disease-related morbidity and biochemical markers of activity in patients with acromegaly. J Clin Endocrinol Metab 90:1972–1978
Reyes-Vidal C, Fernandez JC, Bruce JN, Crisman C, Conwell IM, Kostadinov J, Geer EB, Post KD, Freda PU (2014) Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J Clin Endocrinol Metab 99(11):4124–4132
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22(9):1462–1470
Freda PU, Shen W, Reyes-Vidal CM, Geer EB, Arias-Mendoza F, Gallagher D, Heymsfield SB (2009) Skeletal muscle mass in acromegaly assessed by magnetic resonance imaging and dual-photon X-ray absorptiometry. J Clin Endocrinol Metab 94(8):2880–2886
Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, Shen W, Freda PU, Heymsfield SB (2004) Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol 97(2):655–660
Niculescu D, Purice M, Coculescu M (2013) Insulin-like growth factor-I correlates more closely than growth hormone with insulin resistance and glucose intolerance in patients with acromegaly. Pituitary. 16(2):168–174
Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R (1986) Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol 250(3 Pt 1):E269–273
Karlander S, Vranic M, Efendic S (1986) Increased glucose turnover and glucose cycling in acromegalic patients with normal glucose tolerance. Diabetologia 29(11):778–783
Freda PU, Shen W, Heymsfield SB, Reyes-Vidal CM, Geer EB, Bruce JN, Gallagher D (2008) Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab 93(6):2334–2343
Clemmons DR (2004) The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 113(1):25–27
Clemmons DR (2002) Roles of insulin-like growth factor-I and growth hormone in mediating insulin resistance in acromegaly. Pituitary 5(3):181–183
Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest. 106(4):473–481
Ciresi A, Amato MC, Pivonello R, Nazzari E, Grasso LF, Minuto F, Ferone D, Colao A, Giordano C (2013) The metabolic profile in active acromegaly is gender-specific. J Clin Endocrinol Metab 98(1):E51–59
O’Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK (1994) Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab 78(2):381–386
Bengtsson BA, Brummer RJ, Eden S, Bosaeus I (1989) Body composition in acromegaly. Clin Endocrinol (Oxf) 30(2):121–130
Brummer RJ, Lonn L, Kvist H, Grangard U, Bengtsson BA, Sjostrom L (1993) Adipose tissue and muscle volume determination by computed tomography in acromegaly, before and 1 year after adenomectomy. Eur J Clin Invest 23(4):199–205
Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS (2007) Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am 36(1):75–87
Chaves VE, Junior FM, Bertolini GL (2013) The metabolic effects of growth hormone in adipose tissue. Endocrine 44(2):293–302
Moller N, Schmitz O, Joorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J (1992) Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab 74(5):1012–1019
Sucunza N, Barahona MJ, Resmini E, Fernandez-Real JM, Farrerons J, Lluch P, Puig T, Wagner AM, Ricart W, Webb SM (2008) Gender dimorphism in body composition abnormalities in acromegaly: males are more affected than females. Eur J Endocrinol 159(6):773–779
Lin E, Wexler TL, Nachtigall L, Tritos N, Swearingen B, Hemphill L, Loeffler J, Biller BM, Klibanski A, Miller KK (2012) Effects of growth hormone deficiency on body composition and biomarkers of cardiovascular risk after definitive therapy for acromegaly. Clin Endocrinol (Oxf) 77(3):430–438
Bengtsson BA, Brummer RJ, Eden S, Bosaeus I, Lindstedt G (1989) Body composition in acromegaly: the effect of treatment. Clin Endocrinol (Oxf) 31(4):481–490
Madeira M, Neto LV, de Lima GA, Moreira RO, de Mendonca LM, Gadelha MR, Farias ML (2010) Effects of GH-IGF-I excess and gonadal status on bone mineral density and body composition in patients with acromegaly. Osteoporos Int 21(12):2019–2025
Pirlich M, Schutz T, Ockenga J, Biering H, Gerl H, Schmidt B, Ertl S, Plauth M, Lochs H (2003) Improved assessment of body cell mass by segmental bioimpedance analysis in malnourished subjects and acromegaly. Clin Nutr 22(2):167–174
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96(9):2898–2903
Gibney J, Wolthers T, Burt MG, Leung KC, Umpleby AM, Ho KK (2007) Protein metabolism in acromegaly: differential effects of short- and long-term treatment. J Clin Endocrinol Metab 92(4):1479–1484
Loyman TG, Chen Z (2005) Dual energy X-ray absorptiometry. In: Heymsfield, S, Lohman TG, Wang Z, Going SB (eds) Human body composition. Human Kinetics, Champaign, IL, p 63–78
Ho KK, Gibney J, Johannsson G, Wolthers T (2006) Regulating of growth hormone sensitivity by sex steroids: implications for therapy. Front Horm Res 35:115–128
Jorgensen JO, Christensen JJ, Vestergaard E, Fisker S, Ovesen P, Christiansen JS (2005) Sex steroids and the growth hormone/insulin-like growth factor-I axis in adults. Horm Res 64(Suppl 2):37–40
Parkinson C, Ryder WD, Trainer PJ (2001) The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab 86(11):5240–5244
Gibney J, Healy ML, Sonksen PH (2007) The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev 28(6):603–624
Gibney J, Wolthers T, Johannsson G, Umpleby AM, Ho KK (2005) Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol Endocrinol Metab 289(2):E266–271
Johannsson G, Gibney J, Wolthers T, Leung KC, Ho KK (2005) Independent and combined effects of testosterone and growth hormone on extracellular water in hypopituitary men. J Clin Endocrinol Metab 90(7):3989–3994
Damjanovic SS, Petakov MS, Raicevic S, Micic D, Marinkovic J, Dieguez C, Casanueva FF, Popovic V (2000) Serum leptin levels in patients with acromegaly before and after correction of hypersomatotropism by trans-sphenoidal surgery. J Clin Endocrinol Metab 85(1):147–154
Miyakawa M, Tsushima T, Murakami H, Isozaki O, Demura H, Tanaka T (1998) Effect of growth hormone (GH) on serum concentrations of leptin: study in patients with acromegaly and GH deficiency. J Clin Endocrinol Metab 83(10):3476–3479
Silha JV, Krsek M, Hana V, Marek J, Jezkova J, Weiss V, Murphy LJ (2003) Perturbations in adiponectin, leptin and resistin levels in acromegaly: lack of correlation with insulin resistance. Clin Endocrinol (Oxf) 58(6):736–742
Bolanowski M, Milewicz A, Bidzinska B, Jedrzejuk D, Daroszewski J, Mikulski E (2002) Serum leptin levels in acromegaly—a significant role for adipose tissue and fasting insulin/glucose ratio. Med Sci Monit 8(10):CR685–689
Vilar L, Naves LA, Costa SS, Abdalla LF, Coelho CE, Casulari LA (2007) Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 13(4):363–372
Boero L, Manavela M, Merono T, Maidana P, Gomez Rosso L, Brites F (2012) GH levels and insulin sensitivity are differently associated with biomarkers of cardiovascular disease in active acromegaly. Clin Endocrinol (Oxf) 77(4):579–585
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW (1999) C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19(4):972–978
Derfalvi B, Igaz P, Fulop KA, Szalai C, Falus A (2000) Interleukin-6-induced production of type II acute phase proteins and expression of junB gene are downregulated by human recombinant growth hormone in vitro. Cell Biol Int 24(2):109–114
Carmichael JD, Bonert VS, Mirocha JM, Melmed S (2009) The utility of oral glucose tolerance testing for diagnosis and assessment of treatment outcomes in 166 patients with acromegaly. J Clin Endocrinol Metab 94(2):523–527
Acknowledgments
Funded by NIH Grants R01 DK 064720 and K24 DK 073040 to P.U.F., and in part by Columbia University’s CTSA Grant No. UL1 RR 024156 from NCRR/NIH, an Investigator Initiated Grant from Novartis Pharmaceuticals and a gift from Dr. and Mrs. Leo Guthart.
Conflict of interest
P.U.F. has received research Grant support from Novartis Pharmaceuticals. T.J.R, Z.J., W.S., C.M.R-V., JC.F., J.N.B, J.K., K.D.P. have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trials #: NCT01809808.
Rights and permissions
About this article
Cite this article
Reid, T.J., Jin, Z., Shen, W. et al. IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary 18, 808–819 (2015). https://doi.org/10.1007/s11102-015-0657-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0657-2