Abstract
Aims
GCK-MODY leads to mildly elevated blood glucose typically not requiring therapy. It has been described in all ethnicities, but mainly in Caucasian Europeans. Here we describe our US cohort of GCK-MODY.
Methods
We examined the rates of detection of heterozygous mutations in the GCK gene in individuals referred to the US Monogenic Diabetes Registry with a phenotype consistent with GCK-MODY. We also assessed referral patterns, treatment and demography, including ethnicity, of the cohort.
Results
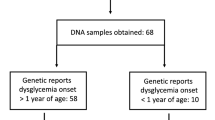
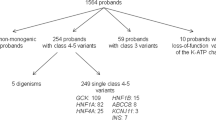
Deleterious heterozygous GCK mutations were found in 54.7 % of Registry probands selected for GCK sequencing for this study. Forty-nine percent were previously unnecessarily treated with glucose-lowering agents, causing hypoglycemia and other adverse effects in some of the subjects. The proportion of probands found to have a GCK mutation through research-based testing was similar across each ethnic group. However, together African-American, Latino and Asian subjects represented only 20.5 % of screened probands and 17.2 % of those with GCK-MODY, despite higher overall diabetes prevalence in these groups.
Conclusions
Our data show that a high detection rate of GCK-MODY is possible based on clinical phenotype and that prior to genetic diagnosis, a large percentage are inappropriately treated with glucose-lowering therapies. We also find low minority representation in our Registry, which may be due to disparities in diagnostic diabetes genetic testing and is an area needing further investigation.
Similar content being viewed by others
References
Chakera AJ, Spyer G, Vincent N et al (2014) The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the atlantic diabetes in pregnancy cohort. Diabetes Care. doi:10.2337/dc13-2248
Sagen JV, Odili S, Bjørkhaug L et al (2006) From clinicogenetic studies of maturity-onset diabetes of the young to unraveling complex mechanisms of glucokinase regulation. Diabetes 55:1713–1722. doi:10.2337/db05-1513
Negahdar M, Aukrust I, Molnes J et al (2014) GCK-MODY diabetes as a protein misfolding disease: the mutation R275C promotes protein misfolding, self-association and cellular degradation. Mol Cell Endocrinol 382:55–65. doi:10.1016/j.mce.2013.08.020
Matschinsky FM (2002) Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes 51(Suppl 3):S394–S404. doi:10.2337/diabetes.51.2007.S394
Davis EA, Cuesta-Munoz A, Raoul M et al (1999) Mutants of glucokinase cause hypoglycaemia- and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 42:1175–1186. doi:10.1007/s001250051289
Byrne MM, Sturis J, Clément K et al (1994) Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Investig 93:1120–1130. doi:10.1172/JCI117064
Beer NL, Osbak KK, van de Bunt M et al (2012) Insights into the pathogenicity of rare missense GCK variants from the identification and functional characterization of compound heterozygous and double mutations inherited in cis. Diabetes Care 35:1482–1484. doi:10.2337/dc11-2420
Osbak KK, Colclough K, Saint-Martin C et al (2009) Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat 30:1512–1526. doi:10.1002/humu.21110
Steele AM, Tribble ND, Caswell R et al (2011) The previously reported T342P GCK missense variant is not a pathogenic mutation causing MODY. Diabetologia 54:2202–2205. doi:10.1007/s00125-011-2194-5
Steele AM, Shields BM, Wensley KJ et al (2014) Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA 311:279–286. doi:10.1001/jama.2013.283980
Stride A, Shields B, Gill-Carey O et al (2014) Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia 57:54–56. doi:10.1007/s00125-013-3075-x
Singh R, Pearson ER, Clark PM, Hattersley AT (2007) The long-term impact on offspring of exposure to hyperglycaemia in utero due to maternal glucokinase gene mutations. Diabetologia 50:620–624. doi:10.1007/s00125-006-0541-8
Bacon S, Schmid J, McCarthy A et al (2015) The clinical management of hyperglycemia in pregnancy complicated by maturity-onset diabetes of the young. Am J Obstet Gynecol 213(236):e1–e7. doi:10.1016/j.ajog.2015.04.037
Naylor RN, John PM, Winn AN et al (2014) Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care 37:202–209. doi:10.2337/dc13-0410
Schnyder S, Mullis PE, Ellard S et al (2005) Genetic testing for glucokinase mutations in clinically selected patients with MODY: a worthwhile investment. Swiss Med Wkly 135:352–356
Pinelli M, Acquaviva F, Barbetti F et al (2013) Identification of candidate children for maturity-onset diabetes of the young type 2 (MODY2) gene testing: a seven-item clinical flowchart (7-iF). PLoS ONE 8:e79933. doi:10.1371/journal.pone.0079933
Shields BM, Hicks S, Shepherd MH et al (2010) Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 53:2504–2508. doi:10.1007/s00125-010-1799-4
Velho G, Blanch H, Vaxillaire M et al (1997) Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia 40:217–224. doi:10.1007/s001250050666
Estalella I, Rica I, De Nanclares GP et al (2007) Mutations in GCK and HNF-1α explain the majority of cases with clinical diagnosis of MODY in Spain. Clin Endocrinol (Oxf) 67:538–546. doi:10.1111/j.1365-2265.2007.02921.x
Lorini R, Klersy C, d’Annunzio G et al (2009) Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care 32:1864–1866. doi:10.2337/dc08-2018
American Diabetes Association (ADA) (2015) Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care 38(Suppl):S4–S4. doi: 10.2337/dc15-S003
Porter JR, Rangasami JJ, Ellard S et al (2006) Asian MODY: are we missing an important diagnosis? Diabet Med 23:1257–1260. doi:10.1111/j.1464-5491.2006.01958.x
Greeley SAW, Naylor RN, Cook LS et al (2011) Creation of the Web-based University of Chicago Monogenic Diabetes Registry: using technology to facilitate longitudinal study of rare subtypes of diabetes. J Diabetes Sci Technol 5:879–886
Vionnet N, Stoffel M, Takeda J et al (1992) Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature 356:721–722. doi:10.1038/356721a0
CDC—National Diabetes Statistics Report, 2014—publications—diabetes DDT. http://www.cdc.gov/diabetes/pubs/statsreport14.htm. Accessed 26 Feb 2016
Naylor R, Philipson LH (2011) Who should have genetic testing for maturity-onset diabetes of the young? Clin Endocrinol (Oxf) 75:422–426. doi:10.1111/j.1365-2265.2011.04049.x
Steele AM, Wensley KJ, Ellard S et al (2013) Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS ONE 8:e65326. doi:10.1371/journal.pone.0065326
Shields BM, McDonald TJ, Ellard S et al (2012) The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 55:1265–1272. doi:10.1007/s00125-011-2418-8
Vigersky RA, Fish L, Hogan P et al (2014) The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab 99:3112–3121. doi:10.1210/jc.2014-2257
Carmody D, Bell CD, Hwang JL et al (2014) Sulfonylurea treatment before genetic testing in neonatal diabetes: pros and cons. J Clin Endocrinol Metab 99:E2709–E2714. doi:10.1210/jc.2014-2494
Alkorta-Aranburu G, Carmody D, Cheng YW et al (2014) Phenotypic heterogeneity in monogenic diabetes: the clinical and diagnostic utility of a gene panel-based next-generation sequencing approach. Mol Genet Metab 113:315–320. doi:10.1016/j.ymgme.2014.09.007
Ellard S, Allen HL, De Franco E et al (2013) Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia 56:1958–1963. doi:10.1007/s00125-013-2962-5
Bonnefond A, Philippe J, Durand E et al (2014) Highly sensitive diagnosis of 43 monogenic forms of diabetes or obesity through one-step PCR-based enrichment in combination with next-generation sequencing. Diabetes Care 37:460–467. doi:10.2337/dc13-0698
Pihoker C, Gilliam LK, Ellard S et al (2013) Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab 98:4055–4062. doi:10.1210/jc.2013-1279
Gloyn AL, van de Bunt M, Stratton IM et al (2009) Prevalence of GCK mutations in individuals screened for fasting hyperglycaemia. Diabetologia 52:172–174. doi:10.1007/s00125-008-1188-4
Ellard S, Thomas K, Edghill EL et al (2007) Partial and whole gene deletion mutations of the GCK and HNF1A genes in maturity-onset diabetes of the young. Diabetologia 50:2313–2317. doi:10.1007/s00125-007-0798-6
Herman S, Varga D, Deissler HL et al (2012) Medium-sized deletion in the BRCA1 gene: limitations of Sanger sequencing and MLPA analyses. Genet Mol Biol 35:53–56
Acknowledgments
This work was supported by grants from the American Diabetes Association (1-11-CT-41), the US National Institutes of Health P30DK020595, UL1RR024999, K23DK094866 K12-HS023007-01 and a gift from the Kovler Family Foundation. We also thank all of the wonderful patients and families who have participated in these studies.
Author contributions
The guarantors, D.C. and R.N., conceptualized and designed the study, wrote the manuscript and researched data. C.B., S.B., J.M. and E.T. researched data and reviewed/edited the manuscript. J.H., L.P. and S.G. contributed to the discussion and reviewed/edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 , as revised in 2008 (5).
Human and animal rights
This study was approved by the University of Chicago Institutional Review Board. All participants provided informed consent for study participation.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Federici.
David Carmody and Rochelle N. Naylor have contributed equally to the work and are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carmody, D., Naylor, R.N., Bell, C.D. et al. GCK-MODY in the US National Monogenic Diabetes Registry: frequently misdiagnosed and unnecessarily treated. Acta Diabetol 53, 703–708 (2016). https://doi.org/10.1007/s00592-016-0859-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0859-8