Abstract
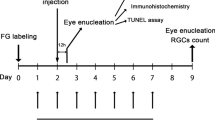
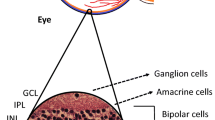
There is now consistent evidence from two major clinical trials (the Fenofibrate Intervention and Event Lowering in Diabetes and the Action to Control Cardiovascular Risk in Diabetes Eye) that fenofibrate arrests the progression of diabetic retinopathy in type 2 diabetic patients. However, the underlying mechanisms of this beneficial effect remain to be elucidated. The aim of the study was to evaluate the potential effect of fenofibric acid (FA), the active metabolite of fenofibrate, in preventing retinal neurodegeneration in an experimental mouse model of type 2 diabetes. For this purpose, we evaluated a total of 24 diabetic mice (db/db) aged 8 weeks that were randomly assigned to daily oral treatment (by gavage) with FA (100 mg/kg/day) (n = 12) or vehicle (n = 12) for 1 week. Ten non-diabetic mice (db/+) were used as control group. Retinal neurodegeneration was evaluated by measuring glial activation (immunofluorescence and Western blot) and apoptosis. Glutamate/aspartate transporter (GLAST) was assessed by immunofluorescence. Functional abnormalities were assessed by electroretinography (ERG). We observed that diabetic mice presented significantly higher glial activation and apoptosis in ganglion cell layer (GCL) than in age-matched non-diabetic mice. Treatment with FA resulted in a significant decrease in both glial activation and the rate of apoptosis in GCL in comparison with diabetic mice treated with vehicle. In addition, FA prevented GLAST downregulation induced by diabetes. Furthermore, a significant improvement of ERG parameters (oscillatory potential amplitudes and b-wave implicit time) was observed. We conclude that FA prevents retinal neurodegeneration induced by diabetes. Our results suggest that neuroprotection is one of the underlying mechanisms by which fenofibrate exerts its beneficial actions in diabetic retinopathy.





Similar content being viewed by others
Abbreviations
- ACCORD:
-
The Action to Control Cardiovascular Risk in Diabetes
- DR:
-
Diabetic retinopathy
- FA:
-
Fenofibric acid
- ERG:
-
Electroretinography
- FIELD:
-
Fenofibrate Intervention and Event Lowering in Diabetes
- GDNF:
-
Glial Cell Line-Derived Neurotrophic Factor
- GCL:
-
Ganglion cell layer
- GLAST:
-
Glutamate–aspartate transporter
- GFAP:
-
Glial fibrillar acidic protein
- H&E:
-
Hematoxylin and eosin
- ISCEV:
-
International Society for Clinical Electrophysiology of Vision
- Lp-PLA2:
-
Lipoprotein-associated phospholipase A2
- OIR:
-
Oxygen-induced retinopathy
- ONL:
-
Outer nuclear layer
- OPs:
-
Oscillatory potentials
- PPAR:
-
Peroxisome proliferator-activated receptor
- RPE:
-
Retinal pigment epithelium
- TUNEL:
-
Terminal Transferase dUTP Nick-End Labeling
References
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136
Mohamed Q, Gillies MC, Wong TY (2007) Management of diabetic retinopathy: a systematic review. JAMA 298:902–916
Simó R, Hernández C (2009) Advances in the medical treatment of diabetic retinopathy. Diabetes Care 32:1556–1562
Rosenson RS (2008) Fenofibrate: treatment of hyperlipidemia and beyond. Expert Rev Cardiovasc Ther 6:1319–1330
Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG, FIELD Study Investigators (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370:1687–1697
ACCORD Study Group, ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363:233–244
Wong TY, Simó R, Mitchell P (2012) Fenofibrate—a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol 154:6–12
Simo R, Roy S, Behar-Cohen F, Keech A, Mitchell P, Wong TY (2013) Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Curr Med Chem 20:3258–3266
Kim J, Ahn JH, Kim JH, Yu YS, Kim HS, Ha J, Shinn SH, Oh YS (2007) Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp Eye Res 84:886–893
Villarroel M, Garcia-Ramírez M, Corraliza L, Hernández C, Simó R (2011) Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1β by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia 54:1543–1553
Trudeau K, Roy S, Guo W, Trudeau K, Roy S, Guo W (2011) Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci 52:6348–6354
Miranda S, González-Rodríguez A, García-Ramírez M, Revuelta-Cervantes J, Hernández C, Simó R, Valverde AM (2012) Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol 227:2352–2362
Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX (2013) Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62:261–272
Barber AJ, Gardner TW, Abcouwer SF (2011) The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci 52:1156–1163
Simó R, Hernández C, European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) (2012) Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br J Ophthalmol 96:1285–1290
Simó R, Hernández C, European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) (2014) Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab 25:23–33
Carrasco E, Hernandez C, Miralles A, Huguet P, Farres J, Simo R (2007) Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 30:2902–2908
Carrasco E, Hernández C, de Torres I, Farrés J, Simó R (2008) Lowered cortistatin expression is an early event in the human diabetic retina and is associated with apoptosis and glial activation. Mol Vis 4:1496–1502
Garcia-Ramírez M, Hernández C, Villarroel M, Canals F, Alonso MA, Fortuny R, Masmiquel L, Navarro A, García-Arumí J, Simó R (2009) Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 52:2633–2641
Barnstable CJ (1993) Glutamate and GABA in retinal circuitry. Curr Opin Neurobiol 3:520–525
Li Q, Puro DG (2002) Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci 43:3109–3116
Bordet R, Ouk T, Petrault O, Gelé P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M (2006) PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans 34(Pt 6):1341–1346
Anderson PJ, Watts H, Hille C, Philpott K, Clark P, Gentleman MC, Jen LS (2008) Glial and endothelial blood-retinal barrier responses to amyloid-beta in the neural retina of the rat. Clin Ophthalmol 2:801–816
Marmor MF, Holder GE, Seeliger MW, Yamamoto S, International Society for Clinical Electrophysiology of Vision (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108:107–114
Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK (2005) Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes 54:3119–3125
Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D (2011) Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 236:1051–1063
Xiao C, He M, Nan Y, Zhang D, Chen B, Guan Y, Pu M (2012) Physiological effects of superoxide dismutase on altered visual function of retinal ganglion cells in db/db mice. PLoS One 7:e30343
Simó R, Bogdanov P, Corraliza L, Carvalho AR, Hernández C (2013) The C57BL/KsJ-db/db mouse: an appropriate model for investigating diabetes-induced retinal neurodegeneration. Diabetologia 56(Suppl 1):S486
Hernández C, García-Ramírez M, Corraliza L, Fernández-Carneado J, Farrera-Sinfreu J, Ponsati B, González-Rodríguez A, Valverde AM, Simó R (2013) Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 62:2569–2578
Wang L, Deng QQ, Wu XH, Yu J, Yang XL, Zhong YM (2013). Upregulation of Glutamate-Aspartate Transporter by Glial Cell Line-Derived Neurotrophic Factor Ameliorates Cell Apoptosis in Neural Retina in Streptozotocin-Induced Diabetic Rats. CNS Neurosci Ther. doi:10.1111/cns.12150
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM (1988) Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 47:815–820
Lieth E, LaNoue KF, Antonetti DA, Ratz M (2000) Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. The Penn State Retina Research Group. Exp Eye 70:723–730
Kowluru RA, Engerman RL, Case GL, Kern TS (2001) Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int 38:385–390
Pulido JE, Pulido JS, Erie JC, Arroyo J, Bertram K, Lu MJ, Shippy SA (2007) A role for excitatory amino acids in diabetic eye disease. Exp Diabetes Res 2007:36150
Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK (1997) Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol 115:1161–1166
Rauen T, Rothstein JD, Wässle H (1996) Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res 286:325–336
Sarthy VP, Pignataro L, Pannicke T, Weick M, Reichenbach A, Harada T, Tanaka K, Marc R (2005) Glutamate transport by retinal Muller cells in glutamate/aspartate transporter-knockout mice. Glia 49:184–196
Deplanque D, Gelé P, Pétrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R (2003) Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci 23:6264–6271
Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, Matsuki N (2001) Phospholipase A2 mediates ischemic injury in the hippocampus: a regional difference of neural vulnerability. Eur J Neurosci 13:2319–2323
Acknowledgments
We thank Abbott for providing us with fenofibric acid (FA). This study was supported by grants from the Ministerio de Ciencia e Innovación (SAF2012-35562), the European Foundation for the Study of Diabetes (EFSD), and the Generalitat de Catalunya (SGR2009-739).
Conflict of interest
None.
Human and animal rights
This article does not contain any studies with human subjects performed by any of the authors. All the animal experiments were performed in accordance with the protocol approved by the Animal Care and Use Committee of Vall d’Hebron Research Institute (VHIR) and Association for Research in Vision and Ophthalmology (ARVO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Federici.
Patricia Bogdanov and Cristina Hernández have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bogdanov, P., Hernández, C., Corraliza, L. et al. Effect of fenofibrate on retinal neurodegeneration in an experimental model of type 2 diabetes. Acta Diabetol 52, 113–122 (2015). https://doi.org/10.1007/s00592-014-0610-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0610-2