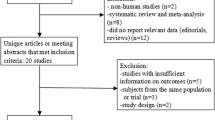
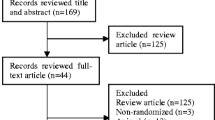
Abstract
Purpose
The aim of this study is to assess the efficacy and tolerability of canagliflozin, a sodium-glucose cotransporter-2 (SGLT-2) inhibitor, added on to metformin in patients with type 2 diabetes mellitus (T2DM).
Methods
Literatures were searched from major electronic databases, as well as the Chinese State Food and Drug Administration and clinicaltrials.gov for unpublished studies. Only randomized controlled trials (RCTs) comparing canagliflozin with placebo in combination with metformin were included. Two reviewers independently selected studies, evaluated the risk of bias, and extracted data. The included RCTs were analyzed by the software RevMan 5.3 provided by the Cochrane Collaboration.
Results
Six RCTs were chosen for the meta-analysis. Compared to placebo, canagliflozin produced absolute reduction in glycated hemoglobin A1c (HbA1c) (−0.66 % [−0.72 %, −0.61 %]). The proportion of patients who achieved target HbA1c was significantly greater in the canagliflozin-treated group (1.86 [1.69, 2.03]). Canagliflozin led to greater fasting plasma glucose (FPG) reduction of 1.49 mmol/L (100 mg/day) and 1.80 mmol/L (300 mg/day). Significant body weight loss of 2.09 % (100 mg/day) and 2.66 % (300 mg/day) with canagliflozin was observed. Canagliflozin was found to improve β cell function in terms of homeostasis model assessment (HOMA2-%B) (15.59 % [12.84 %, 18.35 %]). Higher incidences of genital mycotic infection/female and pollakiuria (increased urine frequency) were noted with canagliflozin compared with placebo-controlled groups.
Conclusions
Canagliflozin is a potential option as an add-on to metformin based on its improvement in HbA1c, FPG, body weight, and β cell function, but further studies are demanded to strengthen this evidence. Common adverse events (AEs) like genital mycotic infection/female and pollakiuria were identified.





Similar content being viewed by others
References
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating G (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378 (9785):31–40.
American Diabetes A (2015) (7) Approaches to glycemic treatment. Diabetes Care 38 Suppl:S41-48.
Brown JB, Conner C, Nichols GA (2010) Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 33(3):501–506
Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future developments in treatment. Lancet 378(9786):182–197
Hardman TC, Dubrey SW (2011) Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther 2(3):133–145
Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54(12):3427–3434
Gerich JE (2010) Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27(2):136–142
Neumiller JJ, White Jr. JR, Campbell RK (2010) Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 70(4):377–385
Nair S, Wilding JP (2010) Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 95(1):34–42
Riser Taylor S, Harris KB (2013) The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy 33(9):984–999
Canagliflozin (Invokana) for type 2 diabetes (2013). Med Lett Drugs Ther 55 (1416):37–39
Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15(4):372–382
Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G (2013) Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 36(9):2508–2515
Polidori D, Zhao Y, Alba M, Ferrannini E (2012) Treatment with canagliflozin (CANA), a sodium glucose co-transporter 2 (SGLT2) inhibitor, for 26 weeks improves indices of betacell function (BCF). Diabetes 61:A265
Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, Desai M, Shaw W, Capuano G, Alba M, Jiang J, Vercruysse F, Meininger G, Matthews D, Group CTC (2015) Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 38(3):403–411
Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, Millington D, Vercruysse F, Canovatchel W, Meininger G (2015) Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 38(3):355–364
Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H (2014) Efficacy and safety of canagliflozin monotherapy in japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, phase III study. Expert Opin Pharmaco 15(11):1501–1515
Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H (2013) Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab 15(12):1136–1145
Inagaki N, Kondo K, Yoshinari T, Kuki H (2015) Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in japanese patients with type 2 diabetes: a 52-week open-label study. J Diabetes Investig 6(2):210–218
Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, Ways K, Schwartz S (2012) Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 14(6):539–545
Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382(9896):941–950
Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K (2013) Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract 41(2):72–84
Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 16(5):467–477
Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, Qiu R, Meininger G (2015) Canagliflozin in asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab 17(1):23–31
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56(12):2582–2592
Qiu R, Capuano G, Meininger G (2014) Efficacy and safety of twice-daily treatment with canagliflozin, a sodium glucose co-transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin and Transl Endocr 1(2):54–60
Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W (2012) Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes care 35(6):1232–1238
Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 67(12):1267–1282
Zhang Q, Dou J, Lu J (2014) Combinational therapy with metformin and sodium-glucose cotransporter inhibitors in management of type 2 diabetes: systematic review and meta-analyses. Diabetes Res Clin Pract 105(3):313–321
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH (2009) The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9:88
Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P (2011) Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 13(7):669–672
Kahn SE (2001) Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86(9):4047–4058
Kahleova H, Mari A, Nofrate V, Matoulek M, Kazdova L, Hill M, Pelikanova T (2012) Improvement in beta-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. J Diabetes Complicat 26(5):442–449
Polidori D, Mari A, Ferrannini E (2014) Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia 57(5):891–901
Diels J, Angermund R, Schroeder M, Worbes-Cerezo M, Thompson G (2014) The efficacy and effectiveness in HbA1c-lowering is dependent on baseline body mass index (BMI) for sitagliptin but not canagliflozin in the treatment of type 2 diabetes mellitus (T2DM). Value Health 17(7):A334
Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, Usiskin K (2014) Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 30(6):1109–1119
Nyirjesy P, Zhao Y, Ways K, Usiskin K (2012) Evaluation of vulvovaginal symptoms and candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 28(7):1173–1178
Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P (2011) Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 13(7):669–672
Yang XP, Lai D, Zhong XY, Shen HP, Huang YL (2014) Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 70(10):1149–1158
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
T.Y. did the literature search and data extraction, selected the studies, carried out the statistical analyses, wrote the manuscript, and contributed to discussion. M.L. did the literature search and selected the studies. L.Y.M. extracted the data and contributed to discussion. Y.Z. selected the studies and contributed to discussion. Y.M.C. contributed to discussion. All authors reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Lu, M., Ma, L. et al. Efficacy and tolerability of canagliflozin as add-on to metformin in the treatment of type 2 diabetes mellitus: a meta-analysis. Eur J Clin Pharmacol 71, 1325–1332 (2015). https://doi.org/10.1007/s00228-015-1923-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1923-y