Abstract
Aims/hypothesis
To assess the impact of impaired insulin secretion (IIS) and insulin resistance (IR) on type 2 diabetes incidence in a Japanese population.
Methods
This 4 year cohort study included 3,059 participants aged 30–69 without diabetes at baseline who underwent comprehensive medical check-ups between April 2006 and March 2007 at Saku Central Hospital. Based on their insulinogenic index and HOMA-IR values, participants were classified by the criteria of the Japan Diabetes Society into four categories: normal; isolated IIS (i-IIS); isolated IR (i-IR); and IIS plus IR. They were followed up until March 2011. The incidence of type 2 diabetes was determined from fasting and 2 h post-load plasma glucose concentrations and from receiving medical treatment for diabetes.
Results
At baseline, 1,550 individuals (50.7%) were classified as normal, 900 (29.4%) i-IIS, 505 (16.5%) i-IR, and 104 (3.4%) IIS plus IR. During 10,553 person-years of follow-up, 219 individuals developed type 2 diabetes, with 126 (57.5%) having i-IIS at baseline. Relative to the normal group, the multivariable-adjusted HRs for type 2 diabetes in the i-IIS, i-IR and IIS plus IR groups were 8.27 (95% CI 5.33, 12.83), 4.90 (95% CI 2.94, 8.17) and 16.93 (95% CI 9.80, 29.25), respectively. The population-attributable fractions of type 2 diabetes onset due to i-IIS, i-IR, and IIS plus IR were 50.6% (95% CI 46.7%, 53.0%), 14.2% (95% CI 11.8%, 15.6%) and 12.9% (95% CI 12.3%, 13.2%), respectively.
Conclusions/interpretation
Compared with IR, IIS had a greater impact on the incidence of type 2 diabetes in a Japanese population.
Similar content being viewed by others
Introduction
The incidence of type 2 diabetes is significantly increasing in Asia, including Japan, China, and Indonesia [1]. Furthermore, Asian Americans have been found to be at significantly higher risk of type 2 diabetes than whites, despite having substantially lower BMI [2]. The mean BMI of patients with type 2 diabetes has been found to differ in human populations, being 32.2 kg/m2 in the USA, 29.4 kg/m2 in the UK and 23.1 kg/m2 in Japan [3–5].
Impaired insulin secretion (IIS) and insulin resistance (IR) are the main pathophysiological components of type 2 diabetes [6–8], with the contributions of these factors thought to differ in Asians and whites. Most white people with type 2 diabetes present with IR [9], which is strongly associated with obesity, whereas most Japanese patients with type 2 diabetes do not have IR [10]. Furthermore, insulin secretion in Japanese individuals is reported to be less than half that in whites [11, 12]. To date, however, few cohort studies have investigated insulin secretion in Asian populations [13, 14]. Because of the importance of investigating the impact of IIS and IR on the development of type 2 diabetes in various ethnic groups, we assessed the impact of both on the development of type 2 diabetes over a median of 4.0 years in a large-scale cohort of Japanese individuals, with insulin secretion and IR determined yearly using standard 75 g OGTTs.
Methods
Study design and participants
The Saku study was designed to determine the incidence of and risk factors for type 2 diabetes among the Japanese population [15]. This study included community residents who underwent comprehensive medical check-ups for the prevention and early detection of various diseases, including diabetes, cardiovascular disease and cancer, at Saku Central Hospital, one of the core hospitals in the Nagano prefecture and located in Saku city of Nagano, Japan. The study involved residents of 15 cities (mainly Saku, Ueda, Nagano, Komoro and Tomi) in the Nagano prefecture who underwent a baseline comprehensive medical check-up over 2 days and 1 night between April 2006 and March 2007. The study protocol was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Saku Central Hospital. Informed consent was obtained from each participant at each examination.
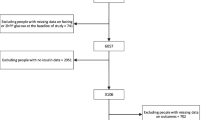
The cohort consisted of 4,318 individuals, aged 30–69 years. Of these, 3,758 did not have diabetes at baseline, based on three criteria: (1) no history of diabetes, as determined by interviews conducted by the physicians; (2) fasting plasma glucose (FPG) concentration <7.0 mmol/l; and (3) 2 h post-load plasma glucose (PG) concentration <11.1 mmol/l. Of these 3,758 individuals, 3,093 (82.3%) underwent at least one follow-up examination by the end of March 2011; we excluded 34 participants with missing data at baseline and/or annual follow-up examinations. Thus, a total of 3,059 individuals (1,754 men and 1,305 women), aged 30–69 years, were eligible for our analysis. Of these, 2,219 (72.5%) underwent comprehensive annual medical check-ups every year for 4 years after the baseline examination.
Procedures
All participants were fasted overnight (12 h), and had a standard 75 g OGTT in the morning. Blood samples were obtained at 0 (fasting), 30, 60 and 120 min, with PG measured on all four occasions and serum insulin concentrations measured at 0 and 30 min in the clinical laboratory of Saku Central Hospital. Serum insulin concentrations were measured using a chemiluminescence enzyme immunoassay (Lumipulse Presto Insulin, Fujirebio, Tokyo, Japan). Blood glucose, HDL-cholesterol, LDL-cholesterol, serum triacylglycerol and uric acid concentrations were measured by enzymatic methods. Leukocyte counts were determined using automated cell counters. High-sensitive C-reactive protein (hsCRP) concentrations were measured using the latex immunity turbidimetric method, and HbA1c concentrations were measured by high-performance liquid chromatography. HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program equivalent value (%) and calculated using the formula HbA1c (%) = HbA1c (Japan Diabetes Society, %) + 0.4% [16]. HbA1c (mmol/mol) was calculated using the formula HbA1c (mmol/mol) = 10.39 × HbA1c (Japan Diabetes Society, %) − 16.8.
Weight, height, waist circumference and body fat percentage were measured in the morning during the fasting state. BMI was calculated as the weight (kg) divided by the height squared (m2). Waist circumference was measured around the abdomen at the level of the navel at the late expiratory phase using a tape measure. Body fat percentage was evaluated by the bioelectric impedance method with an automatic scale. BP was measured by trained nurses using an automatic sphygmomanometer, with the individual in the sitting position after resting for at least 5 min. Each check-up also included standard questionnaires on demographic characteristics, medical history, family history and health-related habits. Alcohol consumption (ethanol) was categorised as 0 g/week, 1–139 g/week, or ≥140 g/week, and exercise was categorised as 0 min/week, 1–119 min/week, or ≥120 min/week.
Definition of IIS and IR
AUCs for type 2 diabetes (FPG ≥7.0 mmol/l and/or 2 h post-load PG ≥11.1 mmol/l) were calculated relative to insulin secretion and IR indices derived from the OGTT in 3,916 participants who were not receiving medical treatment for diabetes at baseline [17–24]. The AUCs of these indices, shown in Table 1, were cross-sectionally evaluated using receiver operating characteristic (ROC) curves. The AUC of the insulinogenic index was highest at 0.825 (95% CI 0.795, 0.855) for insulin secretion, and the AUC of the HOMA-IR was highest at 0.778 (95% CI 0.750, 0.805) for IR.
To determine the optimal cut-offs for the insulinogenic index and HOMA-IR, the Youden index (sensitivity + specificity − 1) was calculated for each, with the optimal cut-off point taken as the value corresponding to the maximum Youden index. The optimal cut-off points for the insulinogenic index and HOMA-IR were 50.4 pmol/mmol (39.0 μU/mg) and 1.7, respectively. These optimal cut-off values were similar to the reference value for IIS (≤51.7 pmol/mmol) and the normal value of HOMA-IR (≤1.6) as defined by the Japan Diabetes Society [13, 25–27]. We therefore defined IIS as an insulinogenic index ≤51.7 pmol/mmol (40.0 μU/mg), and IR as HOMA-IR >1.6. Participants in the study were classified into four categories: normal (insulinogenic index >51.7 pmol/mmol and HOMA-IR ≤1.6); isolated IIS (i-IIS; insulinogenic index ≤51.7 pmol/mmol and HOMA-IR ≤1.6); isolated IR (i-IR; insulinogenic index >51.7 pmol/mmol and HOMA-IR >1.6); and IIS plus IR (insulinogenic index ≤51.7 pmol/mmol and HOMA-IR >1.6).
Definition of type 2 diabetes incidence and follow-up
Type 2 diabetes was defined using the 1999 WHO criteria [28], including FPG ≥7.0 mmol/l and/or 2 h post-load PG ≥11.1 mmol/l, or receiving medical treatment for type 2 diabetes. All 3,059 participants were followed up annually at Saku Central Hospital with comprehensive medical check-ups over 2 days and 1 night, including the 75 g OGTT, until they developed type 2 diabetes or until March 2011. Individuals not examined during follow-up were censored on the date of their last examination.
Statistical analysis
The AUC of the PG (AUCglucose0–120) above fasting level during the OGTT was calculated by the trapezoidal method [29]. Differences in baseline characteristics among the normal, i-IIS, i-IR, and IIS plus IR groups were determined by: analysis of covariance with adjustments for age and sex for normally distributed continuous data; Kruskal–Wallis H tests for non-normally distributed continuous data (triacylglycerol, γ-glutamyltransferase, hsCRP concentrations and insulinogenic index); and χ 2 tests for dichotomous and categorical data.
Cox proportional hazards regression was used to estimate the adjusted HR and 95% CI for the incidence of type 2 diabetes in the i-IIS, i-IR, and IIS plus IR groups relative to the normal group. Multicollinearity between covariates was examined by calculating the mean and individual covariate variance inflation factors. Data were adjusted for age, sex, family history of diabetes (yes or no), current smoking (yes or no), alcohol consumption (0 g/week, 1–139 g/week or ≥140 g/week), and exercise (0 min/week, 1–119 min/week or ≥120 min/week). The assumptions required for proportional hazards were met, and these were assessed with graphs of log–log plots. Population-attributable fraction (PAF) [30, 31] was calculated using the formula: PAF = pd [(HR − 1)/HR], in which pd was the proportion of individuals exposed to a risk factor and HR was the adjusted HR [32]. Multivariable-adjusted HRs and 95% CIs for type 2 diabetes incidence according to major risk factors for diabetes were calculated using Cox proportional hazards regression in the normal, i-IIS, i-IR, and IIS plus IR groups. To test interactions between each of the four groups and each risk factor, each interaction term was added to each model.
Participants were categorised into five groups: individuals who developed type 2 diabetes (i.e. incident cases) in the normal, i-IIS, i-IR and IIS plus IR groups; and individuals who maintained normal glucose regulation (i.e. controls). The log e -transformed insulinogenic index and HOMA-IR trajectories before diabetes onset or at the end of follow-up were calculated for these five groups. The observation period started (year 0) at the year of diabetes onset for incident cases and at the year of the last examination for controls. Participants were then traced backwards through baseline comprehensive medical check-ups. Repeated measurements of log e -transformed insulinogenic index or HOMA-IR were analysed using linear mixed models. The dependent measures were individual log e -transformed insulinogenic index and HOMA-IR values. The fixed effects included time, group, group-by-time, age (covariate) and sex (covariate), and individual was included as a random effect. When there was a significant interaction between group and time, stratified analysis by group was performed. Linear mixed models with Bonferroni adjustment for comparison of the −4 years with the 0 year was used. The dependent measures were individual log e -transformed insulinogenic index or HOMA-IR values. The fixed effects included time, age (covariate) and sex (covariate), and individual was included as a random effect.
All data were analysed using the SAS statistical package software (version 9.1; SAS Institute, Cary, NC, USA) and SPSS statistical software (version 17.0J; SPSS Japan, Tokyo, Japan). All reported p values are two-tailed; those less than 0.05 were considered statistically significant.
Results
Baseline characteristics
The mean age of the 3,059 participants was 55.7 years, and their mean BMI was 23.2 kg/m2. At baseline, 1,550 individuals (50.7%) were classified as normal, 900 (29.4%) as having i-IIS, 505 (16.5%) as having i-IR, and 104 (3.4%) as having both IIS and IR. Table 2 shows the baseline characteristics of the four groups. All variables, except for occupation and exercise, differed significantly among the four groups. Of the four groups, individuals with i-IIS had the lowest BMI, waist circumference, body fat percentage, diastolic BP, LDL-cholesterol concentration, triacylglycerol concentration and leukocyte count, and the highest HDL-cholesterol concentration.
Impact of baseline IIS and IR status on the development of type 2 diabetes
The median follow-up was 4.0 years (total person-years 10,553), during which 219 individuals developed type 2 diabetes, including 26 defined as having type 2 diabetes by receiving medical treatment for this disease. As the youngest of these individuals was 40 years old at baseline, all incident cases were assumed to be type 2 diabetes. Table 3 presents the incidence rates, HRs and PAFs for the development of type 2 diabetes, based on baseline IIS and IR status. The multivariable-adjusted HRs for the incidence of type 2 diabetes in the i-IIS, i-IR, and IIS plus IR groups, relative to the normal group, were 8.27 (95% CI 5.33, 12.83), 4.90 (95% CI 2.94, 8.17) and 16.93 (95% CI 9.80, 29.25), respectively. The PAFs for type 2 diabetes onset due to i-IIS, i-IR and IIS plus IR were 50.6% (95% CI 46.7%, 53.0%), 14.2% (95% CI 11.8%, 15.6%) and 12.9% (95% CI 12.3%, 13.2%), respectively. We observed similar results when we analysed only the 2,219 participants who received annual comprehensive medical check-ups regularly for 4 years after the baseline examination.
Predictors for type 2 diabetes incidence
Table 4 shows multivariable-adjusted HRs for type 2 diabetes in each group during the 4 year follow-up period. Age was a significant predictor of diabetes in the normal and i-IIS groups (p interaction = 0.042), and family history of diabetes was a significant predictor of diabetes in the i-IIS group (p interaction = 0.025). No interaction was observed between overweight/obesity and the four groups (p interaction = 0.289). Sex was not a significant predictor of diabetes in any group.
Trajectories of insulin secretion and IR before type 2 diabetes onset
Figure 1 shows the log e -transformed insulinogenic index and HOMA-IR trajectories before type 2 diabetes onset or at the end of follow-up. The linear mixed model showed significant interactions between group and time for both log e -transformed insulinogenic index (p interaction = 0.021) and HOMA-IR (p interaction < 0.001). Therefore, stratified analysis by group was performed. Incident cases in the i-IIS group showed a steady decrease in insulinogenic index during the 4 years before diabetes onset (−4 vs 0 years: p = 0.012); although their HOMA-IR values were low, they also showed a steady increase in HOMA-IR during the 4 years before diabetes onset (−4 vs 0 years: p = 0.023). Incident cases in the i-IR group showed a steep increase in HOMA-IR values over time (−4 vs 0 years: p < 0.001), whereas the insulinogenic index followed a negative quadratic trajectory during the 4 years before diabetes onset (−4 vs 0 years: p = 0.033).
Log e -transformed insulinogenic index (a) and HOMA-IR (b) trajectories before type 2 diabetes onset or at the end of follow-up among incident cases in the normal (n = 24; black circles), i-IIS (n = 126; black squares), i-IR (n = 39; black triangles) and IIS plus IR (n = 30; black diamonds) groups, and in individuals who maintained normal glucose regulation (n = 2,840; white circles). Repeated measurements of log e -transformed insulinogenic index and HOMA-IR were analysed using a linear mixed model with Bonferroni adjustment for comparison of the −4 years with the 0 year. Each point represents the age- and sex-adjusted mean and the error bars represent 95% CI; 5 pmol/mmol (log e -transformed insulinogenic index) = 150 pmol/mmol (insulinogenic index); 4 pmol/mmol = 55 pmol/mmol; 3 pmol/mmol = 20 pmol/mmol. * p < 0.05 and *** p < 0.001 for −4 vs 0 years
Subcohort with normal glucose regulation
Electronic supplementary material (ESM) Tables 1, 2 and 3 and Fig. 1 shows the results of subcohorts with normal glucose regulation (FPG <6.1 mmol/l and 2 h PG <7.8 mmol/l) [23]. At baseline, 1,338 individuals (58.3%) were classified as normal, 609 (26.5%) as having i-IIS, 310 (13.5%) as having i-IR and 39 (1.7%) as having both IIS and IR. During 8,055 person-years of follow-up, 54 individuals developed type 2 diabetes, with 29 (53.7%) having i-IIS at baseline. Relative to the normal group, the multivariable-adjusted HRs for type 2 diabetes in the i-IIS, i-IR, and IIS plus IR groups were 6.21 (95% CI 2.92, 13.22), 5.16 (95% CI 2.08, 12.78) and 19.69 (95% CI 6.70, 57.83), respectively.
Discussion
This large-scale, community-based cohort study clearly shows the impact of IIS and IR on the incidence of type 2 diabetes in a Japanese population. Our main finding was that, after adjusting for confounding factors, the HRs for type 2 diabetes were 8.27 in the i-IIS group, 4.90 in the i-IR group and 16.93 in the IIS plus IR group when compared with the normal group. Notably, 1.8-fold more individuals in our cohort had i-IIS than i-IR at baseline. In addition, of the 219 incident cases, 126 (57.5%) had i-IIS at baseline. Thus, the PAF (50.6%) of type 2 diabetes onset due to i-IIS was higher than that due to i-IR or IIS plus IR.
Our findings indicate that IIS had a strong impact on the development of type 2 diabetes in this Japanese population. Approximately 50% of cases of incident type 2 diabetes in our population could be attributed to i-IIS, a finding that may explain, at least in part, the high prevalence of type 2 diabetes in Asians with low BMI. Individuals in our i-IIS group were characterised by low BMI and small waist circumference. Moreover, overweight/obesity was not a predictor of diabetes in our i-IIS group. Predictors of diabetes in the i-IIS group were age and family history of diabetes. Variants in the KCNQ1 gene have been reported to increase the risk of future type 2 diabetes, mostly in Asian individuals [33, 34]. The relative risk of type 2 diabetes for Japanese carriers of the KCNJ15 risk allele was 1.76, increasing to 2.54 in individuals with a BMI less than 24 kg/m2 [35]. These KCNQ1 and KCNJ15 variants have been reported to affect the development of type 2 diabetes by impairing beta cell function [33, 34, 36, 37]. These findings suggest that the number of individuals with IIS may be high in Asian populations and that the development of type 2 diabetes in many Asians may be due to IIS.
Approximately 15% of the incidence of type 2 diabetes in our cohort could be attributed to i-IR and approximately 13% to IIS plus IR. Although the PAF of type 2 diabetes onset due to i-IR or IIS plus IR was lower than that due to i-IIS, we found that individuals in our i-IR and IIS plus IR groups had high BMI, waist circumference, body fat percentage, systolic BP, diastolic BP and leukocyte counts, and high concentrations of triacylglycerol, LDL-cholesterol, γ-glutamyltransferase, uric acid and hsCRP. Therefore, additional studies are needed to determine the impact of IIS and IR on the risk for macrovascular and microvascular complications.
There were significant interactions between group and time in both log e -transformed insulinogenic index and HOMA-IR: i.e. the patterns of the trajectories of their insulinogenic index and HOMA-IR differed in the five groups. In incident cases in the i-IIS group, HOMA-IR values were low, but a low insulin-secreting ability was unlikely to compensate for a slight increase in IR. The trajectories of the insulinogenic index and HOMA-IR in incident cases in the i-IIS group may be a pattern specific to Asian populations. In contrast, incident cases in the i-IR group showed a pattern similar to that of the Whitehall II Study, conducted in the UK [38]. Additional long-term studies are needed to determine the natural history of insulin secretion and IR in Asian populations.
In normoglycaemic individuals, the HRs for type 2 diabetes were 6.21 in the i-IIS group, 5.16 in the i-IR group and 19.69 in the IIS plus IR group when compared with the normal group. IR has been found to predict diabetes risk only in normoglycaemic individuals with family history of diabetes, and not in those without [39]. In the present study, although IR was a risk factor for type 2 diabetes in both normoglycaemic individuals with family history of diabetes and those without, the HR for IR was higher in those with a family history of diabetes than in those without.
The strengths of the present study include its large-scale, community-based cohort, which consisted of residents of many cities throughout Nagano prefecture. Furthermore, we screened all participants for type 2 diabetes every year for 4 years using the 75 g OGTT. The 12 h overnight fast before OGTT was managed by hospitalising participants the day before the test.
This study, however, also had several limitations. First, the estimates of insulin secretion and IR were made using calculations based on the OGTT, not by the ‘gold standard’ test, the glucose-clamp technique. However, clamping is not feasible in large-scale epidemiological studies, and we believe that proxy measures are reliable in large datasets. In cross-sectional ROC analysis, the AUCs for the insulinogenic index and HOMA-IR were 0.825 and 0.778, respectively. The insulinogenic index has been used previously in clinical studies [13, 17, 40], and HOMA-IR has also been extensively validated [41, 42].
Second, there may have been a possibility of selection bias, as the participants in this study were individuals who underwent routine comprehensive medical check-ups. Although these check-ups are generally expensive in Japan, those at Saku Central Hospital are relatively inexpensive or free, because administrations and employers subsidise their costs. Therefore, many community residents undergo these examinations, and the rates of diabetes and overweight/obesity in our cohort were similar to those observed in the general Japanese population [43]. Third, individuals who did not undergo annual comprehensive medical check-ups after their baseline examinations were excluded from our analysis. As individuals who developed severe diseases or died during the follow-up period were not assessed, selection bias is possible. However, baseline characteristics such as age, sex, BMI, FPG, 2 h PG, and IIS and IR status did not differ significantly between individuals who were eligible for our analysis and individuals who did not undergo comprehensive medical check-ups after their baseline examinations (data not shown).
In conclusion, this study showed clear evidence of the impact of IIS and IR on the incidence of type 2 diabetes and the trajectories of insulin secretion and IR before type 2 diabetes onset in a Japanese population. The PAF of type 2 diabetes onset due to i-IIS was the highest (50.6%), indicating that IIS had a strong impact on the incidence of type 2 diabetes in this population. As Japanese individuals have low insulin-secreting ability [11], assessments of IIS are necessary so that groups at high risk of developing type 2 diabetes are not overlooked. Our findings may also be applicable to other Asian populations with similar genetic background.
Abbreviations
- AUCglucose0–120 :
-
AUC of the plasma glucose
- FPG:
-
Fasting plasma glucose
- hsCRP:
-
High-sensitive C-reactive protein
- i-IIS:
-
Isolated impaired insulin secretion
- IIS:
-
Impaired insulin secretion
- i-IR:
-
Isolated insulin resistance
- IR:
-
Insulin resistance
- PAF:
-
Population-attributable fraction
- PG:
-
Plasma glucose
- ROC:
-
Receiver operating characteristic
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Lee JW, Brancati FL, Yeh HC (2011) Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 34:353–357
Nelson KM, Reiber G, Boyko EJ (2002) Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 25:1722–1728
Davis TM, Cull CA, Holman RR, U.K. Prospective Diabetes Study (UKPDS) Group (2001) Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the 9 years of type 2 diabetes: U.K. Prospective Diabetes Study (UKPDS 55). Diabetes Care 24:1167–1174
Sone H, Katagiri A, Ishibashi S et al (2002) Effects of lifestyle modifications on patients with type 2 diabetes: the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year-interim report. Horm Metab Res 34:509–515
Kahn SE (2003) The relative contribution of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR (1992) Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340:925–929
Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR (1990) Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113:909–915
Welch S, Gebhart SSP, Bergman RN (1990) Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 71:1508–1518
Taniguchi A, Nakai Y, Fukushima M et al (1992) Pathogenic factors responsible for glucose intolerance in patients with NIDDM. Diabetes 41:1540–1546
Fukushima M, Usami M, Ikeda M et al (2004) Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism 53:831–835
Tripathy D, Carlsson M, Almgren P et al (2000) Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 49:975–980
Kosaka K, Kuzuya T, Yoshinaga H, Hagura R (1996) A prospective study of health check examinees for the development of non-insulin-dependent diabetes mellitus: relationship of the incidence of diabetes with the initial insulinogenic index and degree of obesity. Diabet Med 13:S120–S126
Ito C (1990) Influence of obesity on glucose tolerance and IRI response. Diabetes Res Clin Pract 10:S231–S237
Morimoto A, Tatsumi Y, Deura K, Mizuno S, Ohno Y, Watanabe S (2012) Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men: The Saku Study. J Diabetes Invest. doi:10.1111/jdi.12019
Kashiwagi A, Kasuga M, Araki E et al (2012) International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 3:39–40
Seltzer HS, Allen EW, Herron AL Jr, Brennan MT (1967) Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46:323–334
Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H (1976) Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes 25:241–244
Wareham NJ, Phillips DIW, Byrne CD, Hales CN (1995) The 30 minute insulin increment response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 12:931
Hanson RL, Pratley RE, Bogardus C et al (2000) Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 151:190–198
Ahrén B, Larsson H (2002) Quantification of insulin secretion in relation to insulin sensitivity in nondiabetic postmenopausal women. Diabetes 51:S202–S211
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA (2007) Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30:89–94
Katz A, Nambi SS, Mather K et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Nakai Y, Nakaishi S, Kishimoto H et al (2002) The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med 19:346–347
The Japan Diabetes Society (2010) Evidence-based Practice Guideline for the Treatment of Diabetes in Japan 2010. Nankodo, Tokyo [guideline in Japanese]
The Japan Diabetes Society (2010) Treatment guide for diabetes 2010. Bunkodo, Tokyo [guide in Japanese]
World Health Organization (1999) Report of a WHO consultation: definition, diagnosis and classification of diabetes mellitus and its complications: Part 1. Diagnosis and classification of diabetes mellitus. Department of Noncommunicable Disease Surveillance. Geneva, WHO
Wolever TM, Jenkins DJ (1986) The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 43:167–172
Winell K, Pietilä A, Niemi M, Reunanen A, Salomaa V (2011) Trends in population attributable fraction of acute coronary syndrome and ischaemic stroke due to diabetes in Finland. Diabetologia 54:2789–2794
Kokubo Y, Kamide K, Okamura T (2008) Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension 52:652–659
Rockhill B, Newman B, Weinberg C (1998) Use and misuse of population attributable fractions. Am J Public Health 88:15–19
Hu C, Wang C, Zhang R et al (2009) Variations in KCNQ1 are associated with type 2 diabetes and beta cell function in a Chinese population. Diabetologia 52:1322–1325
Jonsson A, Isomaa B, Tuomi T et al (2009) A variant in the KCNQ1 gene predicts future type 2 diabetes and mediates impaired insulin secretion. Diabetes 58:2409–2413
Okamoto K, Iwasaki N, Nishimura C et al (2010) Identification of KCNJ15 as a susceptibility gene in Asian patients with type 2 diabetes mellitus. Am J Hum Genet 86:54–64
van Vliet-Ostaptchouk JV, van Haeften TW, Landman GW et al (2012) Common variants in the type 2 diabetes KCNQ1 gene are associated with impairments in insulin secretion during hyperglycaemic glucose clamp. PLoS One 7:e32148
Okamoto K, Iwasaki N, Doi K et al (2012) Inhibition of glucose-stimulated insulin secretion by KCNJ15, a newly identified susceptibility gene for type 2 diabetes. Diabetes 61:1734–1741
Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR (2009) Trajectories of glycemia, insulin sensitivity and insulin secretion preceding the diagnosis of type 2 diabetes: the Whitehall II Study. Lancet 373:2215–2221
Goldfine AB, Bouche C, Parker RA et al (2003) Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci USA 100:2724–2729
Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE, American Diabetes Association GENNID, Group S (2002) β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 51:2170–2178
Bonora E, Targher G, Alberiche M et al (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 23:57–63
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Ministry of Health, Labor and Welfare (2005) National Health and Nutrition Examination Survey [in Japanese]. Available from www.mhlw.go.jp/bunya/kenkou/eiyou07/dl/01-03.pdf, accessed 20 October 2012
Acknowledgements
We thank Ryuichi Kikkawa (Shiga University of Medical Science, Shiga, Japan), Tomonori Okamura (Keio University, Tokyo, Japan) and Yoshihiro Miyamoto (National Cerebral and Cardiovascular Center, Osaka, Japan) for their advice on this paper.
Funding
AM is a recipient of a Grant-in-Aid for Japan Society for the Promotion of Science Fellowship. This study was funded by grants from the Ministry of Health, Labor and Welfare, Japan.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AM, YT, KD, SM and SW designed the study. AM, YT, KD and SM contributed to acquisition of the data. AM, YT, and SM performed the analyses. AM wrote the first draft of the paper, and YT, KD, SM, YO, NM and SW contributed to the interpretation of data and critical revision of the manuscript. All authors were involved in the writing of the manuscript and approved the final version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 64 kb)
ESM Table 1
(PDF 131 kb)
ESM Table 2
(PDF 81 kb)
ESM Table 3
(PDF 94 kb)
Rights and permissions
About this article
Cite this article
Morimoto, A., Tatsumi, Y., Deura, K. et al. Impact of impaired insulin secretion and insulin resistance on the incidence of type 2 diabetes mellitus in a Japanese population: the Saku study. Diabetologia 56, 1671–1679 (2013). https://doi.org/10.1007/s00125-013-2932-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2932-y