Abstract
Aims/hypothesis
Hyperfiltration is widely regarded as a contributing factor to the development of microalbuminuria and progressive nephropathy in type 1 diabetes. However, recent studies have questioned this conclusion.
Methods
To address this conflicting evidence, we examined the association between hyperfiltration and progression to microalbuminuria in 2,318 adults with type 1 diabetes. We also compared the estimated GFR in our diabetic patients with rates observed in 6,247 adults from the Finnish general population, using age- and sex-specific z scores.
Results
The distribution of estimated GFR in adults with type 1 diabetes and normoalbuminuria was not significantly different from that expected in the general population (p = 0.51, Mann–Whitney test). Type 1 diabetic patients with a higher estimated GFR were also no more likely to develop microalbuminuria over a median of 5.2 years of follow-up than those with normal estimated GFR. This was the case regardless of whether hyperfiltration was defined by an absolute threshold, deciles of estimated GFR or a z score, using creatinine- or cystatin-based clearance formulas in men or in women.
Conclusions/interpretation
Together with other studies, these data suggest that creatinine- or cystatin-based estimates of GFR do not predict the development of microalbuminuria in patients with type 1 diabetes. Moreover, in the absence of incipient or overt nephropathy, conventionally determined renal function in patients with type 1 diabetes appears no different from that in the general population. This is hardly surprising, given that these individuals, by all definitions, do not have kidney disease.
Similar content being viewed by others
Introduction
It has previously been reported that in up to one third of individuals with type 1 diabetes estimated GFR or creatinine clearance are higher than the upper normal range of age- and sex-matched healthy controls [1]. Conventionally, the development of nephropathy in type 1 diabetes has been characterised as an increase in estimated GFR, which precedes increasing albuminuria by several years [2]. This so-called ‘hyperfiltration’ is widely regarded as a contributing factor to the development of albuminuria and progressive nephropathy [3, 4]. A meta-analysis of ten small studies following 780 normoalbuminuric patients with type 1 diabetes concluded that the presence of hyperfiltration more than doubled the risk of developing micro- or macroalbuminuria [5]. In addition, several small studies also suggested that hyperfiltration predicts the development of renal ultra-structural changes, including thickening of the glomerular basement membrane [6, 7]. However, the large International Diabetic Nephropathy Study found that glomerular basement membrane width, the fractional volume of mesangium and mesangial matrix accumulation were not correlated with baseline renal function [8]. Moreover, a recent single-centre study of 426 normoalbuminuric patients in long-term follow-up at the Joslin Clinic found that hyperfiltration was not associated with an increased risk of progression to microalbuminuria [9]. To address this potentially conflicting evidence, we present here prospective data from an even larger cohort of 2,318 patients with type 1 diabetes and established normoalbuminuria from the FinnDiane study, a nationwide multi-centre study of Finnish adults with type 1 diabetes. By comparing data from our diabetic cohort with data from 6,247 adults from the Finnish general population using age- and sex-specific z scores, we also explored whether conventionally determined estimated GFR in patients with type 1 diabetes is any different from that observed in the general population.
Methods
Study sample
The FinnDiane study has been described in detail in previous publications [10, 11]. In brief, the study was established at Helsinki University Central Hospital, Finland, in order to study clinical, biochemical, environmental and genetic risk factors of type 1 diabetes and its complications. At 21 university and central hospitals, 33 district hospitals, and 26 primary healthcare centres across Finland, all adults with established type 1 diabetes and attending diabetic and/or renal outpatient clinics were asked to participate in the study, regardless of their duration of diabetes. The Ethics Committees of all participating centres approved the study protocol. As previously published, approximately 78% of diabetic patients from these centres were recruited [12]. Written informed consent was obtained from each patient, and the study was performed in accordance with the Declaration of Helsinki as revised in 2000. For the present study, consecutive patients who had normoalbuminuria at baseline and had been recruited into the FinnDiane prospective cohort between 1995 and 2005 were included (n = 2,318). Normoalbuminuria was defined as urinary albumin excretion <20 μg/min in at least two of three consecutive overnight or 24 h urine collections.
Characteristics of the participants
Details on clinical status, including age at diagnosis, presence and severity of diabetic complications, insulin therapy and other regular medications, were obtained from the attending physician using a standardised questionnaire. Data on smoking habits, alcohol intake, educational level and social class were obtained using a patient questionnaire. Fasting blood samples were obtained for the measurement of HbA1c, lipids and high-sensitivity C-reactive protein. Serum creatinine was measured in a fasting blood sample in central laboratories in Helsinki, Finland and calibrated to the isotope dilution mass spectrometry standard.
Estimating GFR
The GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, which has been shown to be accurate in determining renal function in diabetic patients, particularly within the normal range. For comparison purposes, estimated GFR was also estimated using the 175 Modification of Diet in Renal Disease (MDRD) formula [13] and the cystatin C-based formula, which has previously been validated in diabetic patients [14]. Creatinine clearance was also calculated using the Cockcroft and Gault formula, with and without adjustment for body surface area.
Comparisons with the general population
To determine whether the estimated GFR level for any patient with type 1 diabetes was greater than anticipated for his or her age and sex, renal function was compared with that observed in the Finnish age- and sex-matched general population. Serum samples were obtained from the Health 2000 study, a national, population-based health survey [15]. This survey consists of a randomly drawn, nationally representative sample of adults who attended a comprehensive health examination during the years 2000 and 2001. All 6,247 adults in whom serum creatinine measurements were performed were included in the analysis. Serum creatinine was measured at the central laboratories of the University of Helsinki, as detailed above. GFR was estimated using the CKD-EPI formula, and age- and sex-specific distribution was established. Each individual with diabetes from the FinnDiane study was then assigned a z score based on this distribution.
Defining hyperfiltration
In the academic literature, hyperfiltration has been largely considered as a dichotomous variable, where the threshold for hyperfiltration has ranged from 125 to 140 ml min−1 1.73 m−2, depending on the population studied and the formula used to estimate kidney function. For the purposes of this study, all absolute thresholds between 125 and 140 ml/min were tested using each estimation technique. The top decile of patients (i.e. those above the 90th percentile) in each sex was also identified using each estimation technique. In addition, the possibility of an age- or sex-dependent threshold for hyperfiltration was also modelled by specifically incorporating an anticipated age-related decline in renal function and sex-related differences in renal function with a z score ≥2.0 (≥2 SD above the anticipated mean for age and sex) used to denote hyperfiltration.
Statistical tests
Age- and sex-specific z scores were calculated according to the method of Royston and Wright, based on fractional polynomials and exponential transformation [16] as implemented in the Stata user written module ‘xriml’ [17], using the so-called modulus-exponential-normal (MEN) distribution option (which possesses four variables: M [median], S [scale factor] and two shape variables [G and D]). Initially, age-specific z scores were computed for the Health 2000 cohort and appropriate z scores subsequently computed for the FinnDiane diabetic cohort using the MEN distribution and the same fractional polynomial terms for the M and S variables, with G fixed at 1. Distributions were compared using kernel density plots and the two-sample rank-sum (non-parametric) test.
Progression to microalbuminuria was defined as the time to develop a urinary AER of >20 μg/min on two of three readings assessed from an overnight or a 24 h urine collection by immunoturbidimetry according to International Diabetes Federation guidelines [18]. To evaluate the independent predictors of progression to microalbuminuria in individuals with type 1 diabetes, we used Cox proportional hazards models. Hyperfiltration was entered as a categorical variable using different definitions and estimated GFR calculation formulas. All variables known to be associated with renal progression were included in the final models, along with any variables associated with progression in univariate analyses at p < 0.01. In each case, model selection from candidate variables was accomplished by minimising the Akaike and Bayesian information criteria [19]. The potential for multiple co-linearity was tested using the variance inflation factor and condition number, where a variance inflation factor <10 and a condition number <30 are desirable [20]. Analyses were conducted with Stata statistical software (version 11.0, 2009; StataCorp, College Station, TX, USA) and SPSS software (version 13.0; SPSS, Chicago, IL, USA).
Results
Distribution of estimated GFR in adults with type 1 diabetes and normoalbuminuria
The distribution of GFR as estimated by the CKD-EPI in adults with normoalbuminuria from the FinnDiane cohort is shown in Fig. 1a. In individuals with normoalbuminuria, the median estimated GFR was 103 ml min−1 1.73 m−2. Using the CKD-EPI formula, 10% of patients had an estimated GFR >125 ml min−1 1.73 m−2 (denoting the 90th percentile), while 4.2% had an estimated GFR >130 ml min−1 1.73 m−2 (n = 105). Renal function estimated using the MDRD formula was significantly lower than when estimated using the CKD-EPI formula (Fig. 1b). The median estimated GFR was 92 ml min−1 1.73 m−2, with 10% of patients having an estimated GFR >120 ml min−1 1.73 m−2 (denoting the 90th percentile). However, the number of patients with an estimated GFR >130 ml min−1 1.73 m−2 (4.9%, n = 122) using the MDRD formula was similar to that found with the CKD-EPI formula. Use of cystatin C to estimate renal function produced results similar to those obtained using the CKD-EPI formula (Fig. 1c). The median estimated GFR with this method was 102 ml min−1 1.73 m−2, with 10% of patients having an estimated GFR >123.3 ml min−1 1.73 m−2 (denoting the 90th percentile). Using the cystatin C-based formula, 141 patients had an estimated GFR >130 ml min−1 1.73 m−2 (6.1%); this number was greater than with the CKD-EPI formula (p < 0.001).
The distribution of renal function in 2,318 adults with type 1 diabetes and normoalbuminuria, as estimated from serum creatinine by (a) the CKD-EPI formula, compared with estimations using the 175-MDRD formula (b), the cystatin C-based formula proposed by Macisaac et al. [14] (c) and creatinine clearance per 1.73 m2 (d). The continuous lines represent the non-linear (Gaussian-type) regression lines of the distribution of estimated GFR derived from the CKD-EPI formula
Because many of the previous studies examining the link between hyperfiltration and renal progression in type 1 diabetes have sometimes used the Cockcroft–Gault formula to estimate creatinine clearance, we also performed this calculation, with and without adjustment for body surface area. The median creatinine clearance was 96.2 ml min−1 1.73 m−2. The distribution of creatinine clearance was also Gaussian (Fig. 1d), but systematically lower than GFR estimated by the CKD-EPI formula or using cystatin C. Nonetheless, 172 patients were detected as having creatinine clearance >130 ml min−1 1.73 m−2 (7.4%), more than with any other formula. Without any surface area correction, 407 normoalbuminuric patients with type 1 diabetes (17.5%) had creatinine clearance >130 ml/min using the Cockcroft–Gault formula.
Predictors of elevated estimated GFR
Regardless of the formula used to estimate renal function, individuals in the top decile of estimated GFR or creatinine clearance were more likely to be male, younger and with a smaller stature than individuals with an estimated GFR below the 90th centile (p < 0.001 multivariate analysis). In addition, individuals in the top decile of estimated GFR were more likely to be current smokers, after adjusting for age and sex (Table 1). However, HbA1c or blood pressure levels were not significantly higher in participants with elevated estimated GFR levels, after adjusting for age and sex, than in those with lower estimated GFR levels, regardless of the formula used. HDL levels were modestly lower in those with estimated GFR in the upper decile (Table 1). Individuals with elevated GFR were receiving similar disease management with respect to insulin use and blood pressure- and lipid-lowering therapy, after adjusting for age, sex and body size. Although renin–angiotensin system (RAS) blockade may reduce GFR in selected hyperfiltering patients [21], use of RAS blockers was not associated with the prevalence of elevated estimated GFR, after adjusting for age. The duration of diabetes was not associated with the frequency of elevated estimated GFR (electronic supplementary material [ESM] Fig. 1).
z Scores in patients with diabetes and normoalbuminuria
To determine whether the estimated GFR for any adult with type 1 diabetes was greater than anticipated for his or her age and sex, renal function was compared with that observed in the age- and sex-matched general population using the same formula and standardised creatinine concentration measured in the same clinical laboratory. Each diabetic individual was then assigned an age- and sex-specific z score. Using this approach, the distribution of estimated GFR in patients with type 1 diabetes and normoalbuminuria was not significantly different from that expected in the general population (p = 0.51 two-sample Wilcoxon’s rank-sum [Mann–Whitney] test) (Fig. 2).
In our diabetic cohort, 45 individuals had an estimated GFR ≥2.0 SD above the anticipated mean for age and sex (z score ≥2.0). This was not significantly different from that anticipated in the general population (χ 2 = 0.39). Clinical factors associated with a z score ≥2 .0 in patients with diabetes were stature and smoking (p < 0.05 for both), as in the general population. In addition, HbA1c was also associated with the z score in diabetic patients, such that individuals with poor glycaemic control (HbA1c >10%[86 mmol/mol]) had a higher z score on average than those with HbA1c <10%[86 mmol/mol] (0.2 vs 0.0, p < 0.01) and increased prevalence of a z score >2.0 (6% vs 3%, p < 0.01). The duration of diabetes and patient age were not associated with the z score (ESM Fig. 1).
GFR and the development of microalbuminuria
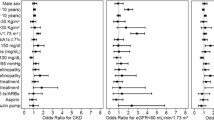
To study the potential role of elevated estimated GFR in the development of nephropathy, the presence or absence of microalbuminuria was determined prospectively in adults with normoalbuminuria and type 1 diabetes at baseline (n = 2,318). During a median of 5.2 years of follow-up, 162 individuals developed microalbuminuria (7.0%). The characteristics of these patients are shown in Table 2. Individuals with a higher estimated GFR were no more likely to develop microalbuminuria than those with a normal value. This was the case, regardless of whether hyperfiltration was defined as an absolute estimated GFR or deciles of estimated GFR in men or in women using the CKD-EPI, MDRD or cystatin C-based estimations (Fig. 3). A z score-based approach offered no additional benefit (Fig. 3). Moreover, when used as a continuous variable, estimated GFR, however estimated, was not associated with the risk of progression, including modelling for non-linear effects with cubic splines (data not shown).
The risk of progression to microalbuminuria in patients with type 1 diabetes and normoalbuminuria in men (a) and women (b), stratified by baseline renal function, and with and without adjustment for duration of diabetes, lipid levels, baseline AER, pre-existing retinopathy, smoking, HbA1c and habitus in multivariate Cox regression analysis. aAdjusted data. eGFR, estimated GFR (ml min−1 1.73 m−2); CrCl, creatinine clearance (ml min−1). Significant HR *p < 0.05
As previously shown in this cohort [22], individuals who did develop microalbuminuria were more likely to be male and have poor glycaemic control, increased waist circumference, dyslipidaemia, background retinopathy and higher AER at baseline (albeit in the normoalbuminuric range) (p < 0.01 for all). After adjusting for the distribution of these risk factors and medication use in multivariate logistic regression analysis, patients who had hyperfiltration (using any threshold, estimation equation or z score) were not more likely to progress from normo- to microalbuminuria than those with renal function in the normal range (Fig. 3). In fact, the risk of progression was significantly reduced in individuals with an MDRD-estimated GFR of >130 ml min−1 1.73 m−2−1 (Fig. 3).
In an additional analysis, all patients with duration of diabetes of less than 5 or more than 25 years were excluded. This was done because it is suggested that it may take at least 10 years to develop diabetic kidney disease and that the incidence of nephropathy may decline after 25 years [2]. Again hyperfiltration, defined as an absolute estimated GFR or deciles of estimated GFR using either the CKD-EPI, MDRD or cystatin C-based estimations, or a z score, was not associated with the risk of progression (data not shown).
Discussion
Hyperfiltration is widely regarded as a contributing factor to the development and progression of renal damage in type 1 diabetes [3, 4]. Although a number of small reports have suggested that elevated estimated GFR in a patient with type 1 diabetes identifies that individual as being at increased risk of developing incipient nephropathy [5, 23], recent studies have disputed these findings [8, 9]. In support of these data, we now show that, in a large well characterised population of adults with type 1 diabetes and normoalbuminuria, elevated estimated GFR identified using conventional tests is not associated with the development of microalbuminuria, after adjusting for other factors, including sex, glycaemic control and an anticipated age-related decline in renal function. Taken together with other recent studies [8, 9], these data suggest that creatinine or cystatin-based estimates of GFR do not predict the development of microalbuminuria in patients with type 1 diabetes.
An increase in the estimated GFR is said to be among the earliest changes observed in the diabetic kidney. In experimental animals, a marked increase in estimated GFR can be observed within hours of developing persistent hyperglycaemia [24]. Patients with newly diagnosed diabetes often present with an elevated estimated GFR [4], which is rapidly normalised by intensive treatment with insulin [4]. It has previously been reported that between 13% and 67% of individuals with type 1 diabetes have an elevated estimated GFR [3]. However, in modern clinical practice, where few patients have persistent hyperglycaemia and all patients have access to insulin and glucose-monitoring technologies, the clinical scenario that led to the initial observations of ‘hyperfiltration’ in diabetic patients may no longer exist. Indeed, our data suggest that in well managed diabetic individuals without albuminuria, the prerequisite for diabetic nephropathy, i.e. renal function estimated using conventional assays and formulas, is no different from that observed in the general population after adjusting for age and sex (Fig. 2). This is perhaps not surprising given that these individuals, by all definitions, do not have kidney disease.
Many previous studies examining the association between hyperfiltration and renal outcomes in patients with type 1 diabetes have used direct measures of renal function, including inulin, iohexol or isotopically labelled markers using a single injection technique. While such techniques are widely considered to be the ‘gold standard’ for estimation of GFR, such studies have been significantly constrained by small patient numbers, selection bias, lack of age- and sex-appropriate non-diabetic reference ranges, and methodological factors, which lead to overestimation of the true GFR, especially in young adults [3]. By contrast, those studies not demonstrating an association between hyperfiltration and renal outcomes [8, 9], including our own, have generally estimated GFR using standardised circulating markers (creatinine and/or cystatin C). Although widely used in the clinical assessment and management of patients with type 1 diabetes, it is possible that such conventional techniques may be less sensitive in identifying ‘hyperfiltering’ patients. Certainly, creatinine-based methods tend to underestimate the ‘true’ GFR, particularly at normal to high levels of estimated GFR and when using the MDRD formula [25, 26]. However, such limitations may be equally applicable when estimating renal function in the general population, where the prevalence of an elevated estimated GFR was not different from that observed in patients with type 1 diabetes.
Until the last decade, creatinine clearance was historically used as the preferred measure of kidney function. This also may have made hyperfiltration appear more common than it really is, especially in young adults. For example, using the Cockcroft–Gault formula without adjusting for surface area, we found that 17.5% of our patients had a creatinine clearance >130 ml/min. This approximates the reported prevalence of ‘hyperfiltration’ in earlier studies, which also used similar cut-offs and clearance formulas [3]. However, we again note that a similar prevalence of creatinine clearance-defined hyperfiltration is found in the age- and sex-matched general population. Moreover, with or without correction for body surface area, creatinine clearance was not associated with an increased risk of developing microalbuminuria in our diabetic patients.
Finally, differences between the previous studies may also have been compounded by variability in the definition of microalbuminuria used. For example, in the six studies [2, 24, 27–30] that used a conventional definition of microalbuminuria, there was no significant association between hyperfiltration and renal progression. In the present study, we also used the internationally recommended definition of microalbuminuria [18] and also found no association between hyperfiltration and progressive kidney disease. In the Joslin Clinic study, which also found no association, progression to microalbuminuria was defined as two consecutive measurements >30 μg/min [9]. By contrast, some of the studies that did demonstrate a significant association between hyperfiltration and progression used unconventional definitions to denote progression.
The present study has a number of strengths, including its consideration of a large number of unselected normoalbuminuric patients, as well as its careful consideration of multiple potential risk factors and their interactions. We chose a pragmatic approach, which involved establishing the normal distribution of estimated GFR in the Finnish general population, thus allowing us to assign an age- and sex-appropriate z score to each diabetic patient in the FinnDiane cohort. Many of the previous studies have been small, of short duration and have selected cases with highly elevated estimated GFR determined by variable direct measures; they have also failed to account for potential confounders, especially age, sex and glycaemic control. For example, it has been argued that uncontrolled hyperglycaemia in some earlier studies included in the meta-analysis [5] (many including patients with a mean HbA1c >10% [86 mmol/mol]) contributed to hyperfiltration and its putative association with progressive kidney disease in diabetic patients [3]. Notably, the Joslin study [9], which did not see any association between hyperfiltration and renal disease progression, achieved significantly lower HbA1c levels than observed in the earlier positive studies included in the previous meta-analysis. In our Finnish cohort, mean HbA1c levels (~9% [55.8 mmol/mol]) lay between those of the previous studies, suggesting that better glycaemic control is not the total explanation of the lack of association between hyperfiltration and renal disease progression observed by us. Nonetheless, glycaemic control was independently associated with estimated GFR and creatinine clearance in our cohort, such that individuals with higher levels of HbA1c had higher estimated GFR, creatinine clearance and z scores than those with better glycaemic control. In addition, poor glycaemic control was associated with an increased incidence of microalbuminuria. However, there were no interactions between glycaemic control, estimated GFR and renal disease progression, implying that, at least statistically, these effects were independent.
In our cohort, the incidence of microalbuminuria was also associated with lipid levels, as previously described [31]. Lipid levels were also associated with estimated GFR, both in the general population and in adults with type 1 diabetes. However, there was no interaction between dyslipidaemia, estimated GFR and the risk of microalbuminuria. Moreover, hyperfiltration was not associated with progression, with or without adjustment for lipid levels. Interestingly, individuals with an elevated estimated GFR in the general population had significantly higher levels of fasting triacylglycerol after adjusting for age, sex and glycaemic control. This association was also independent of BMI, a known predictor of hyperfiltration even in non-obese patients [32, 33]. An independent association between dyslipidaemia and hyperfiltration has also been observed in other populations [34]. However, a precise pathophysiology remains to be established.
The interpretations of outcomes in our study have some limitations. Importantly, the rate of progression to albuminuria was modest in our cohort, with less than 8% of normoalbuminuric patients developing microalbuminuria over 5 years of follow-up. This is less than half of that reported in earlier studies included in the meta-analysis [5], and potentially reflects improving diabetes care and declining rates of nephropathy over the same time period [35]. In studying patients with normoalbuminuria, we excluded those with established microalbuminuria, in whom hyperfiltration may have been more important in the past. In addition, it is possible that there is only a brief window of hyperfiltration, preceding the development of albuminuria, and that this may have been missed in our progressing patients. However, unlike previous studies, we employed a time to event (Cox) regression analysis, which better takes into account the clinical natural history. Finally, we did not include paediatric or adolescent patients, or examine renal function in healthy young Finns. It is possible that hyperfiltration in type 1 diabetes is a purely childhood or early adult phenomenon [23], although no systematic comparison with non-diabetic controls has ever been performed to test this hypothesis, which, in addition, seems unlikely on the basis of the pathophysiology of type 1 diabetes.
In summary, the available clinical data do not support the notion that an elevated estimated GFR, as measured using conventional creatinine or cystatin-based clearance formulas, is a risk factor for the development of microalbuminuria in type 1 diabetes. Moreover, in the absence of incipient or overt nephropathy, renal function in patients with type 1 diabetes appears to be no different from that observed in the general population when using conventional assays. Indeed, elevated estimated GFR appears to be as likely to occur in adults from the general population, should we choose to measure it. By contrast, glycaemic control and lipid levels remain strongly associated with the risk of progressive renal damage, and as such remain the important targets for the prevention of nephropathy in patients with type 1 diabetes.
Abbreviations
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- MDRD:
-
Modification of Diet in Renal Disease
- MEN:
-
Modulus–exponential–normal
- RAS:
-
Renin–angiotensin system
References
Mogensen CE, Steffes MW, Deckert T, Christiansen JS (1981) Functional and morphological renal manifestations in diabetes mellitus. Diabetologia 21:89–93
Caramori ML, Gross JL, Pecis M, de Azevedo MJ (1999) Glomerular filtration rate, urinary albumin excretion rate, and blood pressure changes in normoalbuminuric normotensive type 1 diabetic patients: an 8-year follow-up study. Diabetes Care 22:1512–1516
Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ (2010) The clinical significance of hyperfiltration in diabetes. Diabetologia 53:2093–2104
Wiseman MJ, Saunders AJ, Keen H, Viberti G (1985) Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med 312:617–621
Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG (2009) Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52:691–697
Rudberg S, Osterby R, Dahlquist G, Nyberg G, Persson B (1997) Predictors of renal morphological changes in the early stage of microalbuminuria in adolescents with IDDM. Diabetes Care 20:265–271
Berg UB, Torbjornsdotter TB, Jaremko G, Thalme B (1998) Kidney morphological changes in relation to long-term renal function and metabolic control in adolescents with IDDM. Diabetologia 41:1047–1056
Drummond K, Mauer M (2002) The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51:1580–1587
Ficociello LH, Perkins BA, Roshan B et al (2009) Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care 32:889–893
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46:1402–1407
Saraheimo M, Forsblom C, Thorn L et al (2008) Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care 31:1165–1169
Fagerudd J (2004) Implementation of guidelines for the prevention of diabetic nephropathy. Diabetes Care 27:803–804
No authors listed (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1):S1–S266
Macisaac RJ, Premaratne E, Jerums G (2011) Estimating glomerular filtration rate in diabetes using serum cystatin C. Clin Biochem Rev 32:61–67
Institute NPH (2002) Health 2000: Health and Functional Capacity of Finns: a National Health Survey. National Institute for Health and Welfare, Helsinki
Royston P, Wright EM (1998) A method for estimating age-specific reference intervals (‘normal ranges’) based on fractional polynomials and exponential transformation. J R Stat Soc Ser A-Stat Soc 161:79–101
Wright E, Royston P (1997) Age-specific reference intervals ("normal ranges"). Stata Tech Bull Repr 6:91–107
Force ICGT (2005) Global guideline for type 2 diabetes. International Diabetes Federation, Brussels
Kuha J (2005) AIC and BIC. Comparisons of assumptions and performance. Sociol Methods Res 33:188–229
Belsley D (1991) Conditioning diagnostics, collinearity and weak data in regression. Wiley, New York
Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA (2006) Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17:1703–1709
Vergouwe Y, Soedamah-Muthu S, Zgibor J, Chaturvedi N, Forsblom C (2010) Progression to microalbuminuria in type 1 diabetes: development and validation of a prediction rule. Diabetologia 53:254–262
Amin R, Turner C, van Aken S et al (2005) The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney Int 68:1740–1749
Brenner BM (1983) Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23:647–655
Nair S, Mishra V, Hayden K et al (2011) The four-variable modification of diet in renal disease formula underestimates glomerular filtration rate in obese type 2 diabetic individuals with chronic kidney disease. Diabetologia 54:1304–1307
Stevens LA, Schmid CH, Greene T et al (2010) Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56:486–495
Dahlquist G, Stattin EL, Rudberg S (2001) Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant 16:1382–1386
Zerbini G, Bonfanti R, Meschi F et al (2006) Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 55:2620–2625
Lervang HH, Jensen S, Brochner-Mortensen J, Ditzel J (1992) Does increased glomerular filtration rate or disturbed tubular function early in the course of childhood type 1 diabetes predict the development of nephropathy? Diabet Med 9:635–640
Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G (2008) Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care 31:971–973
Tolonen N, Forsblom C, Thorn L, Waden J, Rosengard-Barlund M, Saraheimo M (2009) Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia 52:2522–2530
Krikken JA, Bakker SJ, Navis GJ (2009) Role of renal haemodynamics in the renal risks of overweight. Nephrol Dial Transplant 24:1708–1711
Chagnac A, Herman M, Zingerman B et al (2008) Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 23:3946–3952
Tantravahi J, Srinivas TR, Johnson RJ (2007) Hyperfiltration: a sign of poor things to come in individuals with metabolic syndrome. Nat Clin Pract Nephrol 3:474–475
Cooper M (2006) Is diabetic nephropathy disappearing from clinical practice. Pediatr Diabetes 7:237–238
Acknowledgements
We acknowledge all the physicians and nurses at each participating centre for their invaluable role in patient recruitment, and collection of samples and data as previously described in detail [10].
Funding
The FinnDiane study is funded by the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Sigrid Juselius Foundation, the European Commission, Medicinska understödsföreningen Liv och Hälsa, Signe and Ane Gyllenberg Foundation, Waldemar von Frenckell Foundation, EVO governmental grants and the NIH. None of these groups had a role in data collection, analysis or preparation of the manuscript.
Contribution statement
VH, LT, JW, MS, NT, JL, AJ and CF were involved in conception and design, data collection and manuscript preparation. MCT, PHG, CF and JLM were involved in conception and design, analysis and interpretation of data, and manuscript preparation. All authors approved the final submitted version.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 305 kb)
Rights and permissions
About this article
Cite this article
Thomas, M.C., Moran, J.L., Harjutsalo, V. et al. Hyperfiltration in type 1 diabetes: does it exist and does it matter for nephropathy?. Diabetologia 55, 1505–1513 (2012). https://doi.org/10.1007/s00125-012-2485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2485-5