Abstract
Aims/hypothesis
To evaluate whether exposure to maternal gestational diabetes (GDM) is associated with adiposity and fat distribution in a multiethnic population of children.
Methods
Retrospective cohort study of 82 children exposed to maternal GDM and 379 unexposed youths 6–13 years of age with measured BMI, waist circumference, skinfold thickness, and visceral and subcutaneous abdominal fat.
Results
Exposure to maternal GDM was associated with higher BMI (p = 0.02), larger waist circumference (p = 0.004), more subcutaneous abdominal fat (p = 0.01) and increased subscapular to triceps skinfold thickness ratio (p = 0.01) in models adjusted for age, sex, race/ethnicity and Tanner stage. Adjustment for socioeconomic factors, birthweight and gestational age, maternal smoking during pregnancy and current diet and physical activity did not influence associations; however, adjustment for maternal pre-pregnancy BMI attenuated all associations.
Conclusions/interpretation
Exposure to maternal GDM is associated with increased overall and abdominal adiposity, and a more central fat distribution pattern in 6- to 13-year-old youths from a multi-ethnic population, providing further support for the fetal overnutrition hypothesis.
Similar content being viewed by others
Introduction
The prevalence of overweight and obesity in children is increasing, and overweight is now present at younger ages [1]. Obesity in childhood and adolescence is associated with long-term consequences, including adult obesity, type 2 diabetes and increased cardiovascular morbidity and mortality.
Exposure to maternal diabetes during intrauterine life leads to increased fetal growth. Fuel-mediated teratogenesis (also called fetal overnutrition) has been one of the suggested mechanisms [2]. Fetal overnutrition may also result in an increased risk for obesity later in life. Solid evidence of an association between exposure to maternal diabetes and childhood obesity has been provided by the Pima Indian Study [3]. This study also provided evidence that the association is likely to be causal and in addition to genetic susceptibility to obesity and diabetes [3]. Less conclusive findings were noted among youths of European descent, in the Growing Up Today Study [4], Project Viva [5] and in the Avon Longitudinal Study of Parents and Children (ALSPAC) [6]. This highlights the need to carefully evaluate the effects of exposure to maternal diabetes on childhood body size among diverse populations. Moreover, previous studies have not examined the effects of exposure to maternal diabetes on more sensitive markers of childhood adiposity and fat distribution. The validity and utility of BMI as a sole measure of body fatness in children, especially when ethnically diverse, are debatable.
The current study assessed the relationship between exposure to maternal gestational diabetes (GDM) and childhood overall adiposity and fat distribution in a multiethnic population of children (6–13 years) that includes non-Hispanic white (NHW), Hispanic and African-American (AA) youths.
Methods
Participants
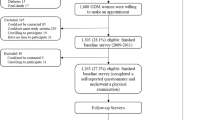
This report uses data from a retrospective cohort study conducted in Colorado: Exploring Perinatal Outcomes among Children (EPOCH). Participants were 6- to 13-year-old offspring of singleton pregnancies, born at a single hospital in Denver between 1992 and 2002, whose biological mothers were members of the Kaiser Permanente of Colorado Health Plan (KPCO), and who were still KPCO members and living in Colorado over the study period.
Eligible participants were children exposed to maternal GDM and a random sample of children not exposed and without intrauterine growth restriction (defined as birthweight for gestational age score < the 10th percentile). Children and their biological mothers were invited for a research visit. The study was approved by the relevant Institutional Review Boards. All participants provided written informed consent and the youths provided written assent.
Exposure definition
GDM status was ascertained from the KPCO perinatal database, an electronic database linking the neonatal and perinatal medical record. GDM is coded as present if diagnosed through the standard KPCO screening protocol and absent if screening was negative. Since the 1990s, KPCO has routinely screened for GDM in all non-diabetic pregnancies using a two-step standard protocol. In addition, birthweight, gestational age, GDM treatment and maternal pre-pregnancy weight were also obtained from the database.
Measures of childhood adiposity and fat distribution
Childhood height and weight were measured in light clothing and without shoes and BMI was calculated as kg/m2. Waist circumference was measured according to the National Health and Nutrition Examination Survey protocol [7]. Skinfold thickness was measured in triplicate using Holtain calipers and averaged. The ratio of subscapular to triceps skinfold thickness (STR) was calculated to assess regional differences in subcutaneous fat distribution. MRI of the abdominal region was used to quantify visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) with a 3 T HDx Imager (General Electric, Waukashau, WI, USA) by a trained technician. Each study participant was placed supine and a series of T1-weighted coronal images were taken to locate the L4/L5 plane. One axial, 10 mm, T1-weighted image, at the umbilicus or L4/L5 vertebrae, was analysed to determine SAT and VAT content. Images were analysed by a single reader, blinded to exposure status.
Other measurements
Race/ethnicity was self-reported using 2000 US Census-based questions and categorised as Hispanic (any race), NHW and AA. Children’s total energy intake (calories/day) was assessed using the Block Kid’s Food Questionnaire. Self-reported key activities performed during the previous 3 days were queried using a 3-day physical activity recall questionnaire. Results were reported as average number of 30 min blocks of moderate-to-vigorous activity per day. Pubertal development was assessed by child self-report with a diagrammatic representation of Tanner staging adapted from Marshall and Tanner, and youths were categorised as Tanner <2 (pre-pubertal) and ≥2 (pubertal). Maternal pre-pregnancy BMI was calculated from the KPCO-measured weight before the last menstrual cycle preceding pregnancy and height collected at the in-person research visit. Maternal level of education, total household income and smoking at any time during pregnancy were self-reported during the research office visit.
Statistical analysis
Multiple linear regression was used to examine the association of exposure to maternal diabetes with measures of offspring adiposity (BMI, waist circumference, VAT, SAT and STR), controlling for potential confounders. A significant interaction between age and Tanner stage on VAT was noted (p = 0.0008), suggesting that the effect of age on childhood adiposity depends on pubertal development. As such an effect was reported previously, an interaction term between age and Tanner stage was included in our base model for all outcomes of interest. Several multiple linear regression models were developed for each outcome. Model 1 (base model) was adjusted for demographic factors: age, sex, race/ethnicity, Tanner stage and the age–Tanner stage interaction term. In models 2–5 we explored the effect of adjustment for potential confounders by adding specific variables to our base model: Model 2 included maternal age at delivery, maternal education and total household income; model 3 added birthweight, gestational age and smoking during pregnancy; model 4 added child’s total daily calories and physical activity; and model 5 included model 1 variables plus maternal pre-pregnancy BMI. Finally, model 6 included all the above variables.
Results
In total, 82 children exposed to GDM and 379 unexposed youths had complete data on variables of interest. All mothers with GDM were diet-treated, while insulin was used in 19 participants (23%). Table 1 shows characteristics of the study population, according to exposure status. Compared with unexposed children, those exposed to GDM were younger, more likely to be NHW and less likely to have begun puberty. Mothers of exposed offspring were older at delivery than those of unexposed offspring, were more likely to smoke during pregnancy and had a higher pre-pregnancy BMI. Exposed and unexposed offspring were not significantly different in terms of current behaviours, intrauterine growth and socioeconomic factors.
Table 2 shows the results of multiple regression analyses for each outcome of interest. Model 1 shows that exposure to GDM was associated with 1.3 kg/m2 higher BMI (p = 0.02), 4.2 cm larger waist circumference (p = 0.004), 3.6 cm2 more VAT (p = 0.1), 34.7 cm2 more SAT (p = 0.01) and a more centralised fat distribution (STR 0.83 vs 0.76, p = 0.01), independent of age, sex, race/ethnicity and Tanner stage. Models 2–4 show no substantial effect of additional adjustment for socioeconomic factors (model 2), markers of intrauterine growth (model 3) and current behavioural factors (model 4). On adjustment for maternal pre-pregnancy BMI (model 5), associations between exposure to gestational diabetes and adiposity outcomes were substantially, but not completely, attenuated. Two additional tables provided online show the association between adiposity outcomes and various maternal and childhood characteristics of interest (Electronic supplementary material [ESM] Table 1) and the association between exposure to diabetes in utero and adiposity outcomes, stratified by NHW and Hispanic race/ethnicity (ESM Table 2).
Discussion
We found that exposure to maternal GDM is associated with higher BMI, waist circumference, VAT, SAT and a more centralised fat distribution pattern in 6- to 13-year-old multiethnic youths from Colorado. Our results substantially add to the growing body of evidence in this area. Several [3, 8, 9] but not all [5, 10] studies have found an association between exposure to maternal GDM and offspring BMI or overall obesity. Surprisingly, in a recent follow-up study of a randomised clinical trial in Australia, treatment of mild GDM did not result in BMI differences in offspring aged 4–5 years old [11]. The discrepant results between various studies may be due to differences in the age at which these offspring were examined, and have generated the hypothesis that the long-term effects of GDM exposure on childhood obesity may not become apparent until later during childhood (e.g. during puberty).
To our knowledge, this is the first to address the effect of GDM exposure on childhood abdominal adiposity and fat distribution using state of the art techniques. While the natural evolution of fat formation and deposition in children requires further study, it appears that abdominal obesity in pre-pubertal children is mainly subcutaneous, while levels of visceral fat increase with increasing age and puberty [12]. We found that exposure to GDM was associated with increases in both subcutaneous and visceral adiposity; however, given the young age of these children, the main abdominal depots were subcutaneous. Similarly, although STRs were on average <1 in both exposed and unexposed offspring, indicating a fat distribution pattern characteristic of pre-pubertal youth, exposure to GDM was significantly associated with an increased ratio of central to peripheral fat deposition. Similar findings were reported by Krishnaveni among 9-year-old Indian children [13] and Project Viva among 3-year-old children in Boston [5]. Together with our data, these findings suggest that GDM exposure may have a stronger influence on abdominal adiposity and fat patterning than on overall obesity, at least during early childhood.
We found that the association between GDM exposure and childhood adiposity was independent of birthweight, suggesting, as in previous studies, that the long-term consequences of fetal overnutrition are not completely mediated through increased fetal growth. In our study, there was no effect of adjustment for socioeconomic factors, possibly because this was a highly educated, relatively wealthy population. Similarly, adjustment for measures of childhood diet and physical activity had no substantial influence on most relationships of interest. More research is needed to understand whether healthy postnatal lifestyles may modify the long-term consequences of exposure to GDM.
Similarly to other studies [4, 6], adjustment for maternal pre-pregnancy BMI attenuated all associations; however, substantial differences remained for waist circumference, SAT and STR, even after adjustment. Adjustment for maternal pre-pregnancy BMI may partly control for a competing mechanism, i.e. genetic predisposition to obesity. Alternatively, maternal obesity may also be part of the fetal overnutrition pathway [4], in which this case may result in overadjustment. Only 23% of mothers with GDM were treated with insulin during their pregnancy. Adjustment for GDM treatment did not influence our findings (data not shown).
Our study has several limitations. The young age of our cohort may have resulted in smaller differences in various adiposity variables according to exposure status. Prospective follow-up of this cohort is necessary to better understand the effect of exposure to maternal diabetes on development of childhood adiposity. The age range of this cohort was relatively large, and adjustment for age and Tanner stage was necessary. Owing to the relatively small sample size, race/ethnic-specific analyses were underpowered; however, the patterns of associations were consistent among all racial/ethnic groups and tended to be strongest among Hispanic children (data not shown). Our study has also very important strengths including a validated exposure assessment, assessed without concern for recall bias, state of the art measures of childhood adiposity, and an ethnically diverse group of offspring.
In conclusion, we provide additional evidence that intrauterine exposure to maternal diabetes is associated with childhood adiposity in a diverse population of children. These findings provide further support to the fetal overnutrition hypothesis and suggest that prevention of childhood obesity needs to start early in life, possibly by targeting the increasing number of women affected by diabetes during childbearing years.
Abbreviations
- AA:
-
African-American
- EPOCH:
-
Exploring Perinatal Outcomes among Children
- GDM:
-
Gestational diabetes mellitus
- KPCO:
-
Kaiser Permanente of Colorado
- NHW:
-
Non-Hispanic white
- SAT:
-
Subcutaneous adipose tissue
- STR:
-
Subscapular/triceps skinfold thickness ratio
- VAT:
-
Visceral adipose tissue
References
Dubois L, Girard M (2006) Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obesity 30:610–617
Freinkel N (1980) Banting Lecture 1980. Of pregnancy and progeny. Diabetes 29:1023–1035
Dabelea D, Hanson RL, Lindsay RS et al (2000) Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49:2208–2211
Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA (2003) Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111:e221–e226
Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E (2009) Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 22:215–220
Lawlor DA, Fraser A, Lindsay RS et al (2010) Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53:89–97
National Health and Nutrition Examination Survey (NHANES) (2007) Anthropometry procedures manual. Centers for Disease Control and Prevention
Boney CM, Verma A, Tucker R, Vohr BR (2005) Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115:e290–e296
Egeland GM, Meltzer SJ (2010) Following in mother’s footsteps? Mother–daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet Med 27:257–265
Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH (1998) Gestational diabetes and the risk of offspring obesity. Pediatrics 101:E91–E97
Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA (2010) Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diab Care 33:964–968
Fox KR, Peters DM, Sharpe P, Bell M (2000) Assessment of abdominal fat development in young adolescents using magnetic resonance imaging. Int J Obes Relat Metab Disord 24:1653–1659
Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH (2010) Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diab Care 33:402–404
Acknowledgements
This work was supported by RO1 DK068001(D. Dabelea) and by M01 RR00069 (General Clinical Research Centers Program).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript. The study sponsor had no role in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Relationships between childhood adiposity outcomes and relevant offspring and maternal characteristics (PDF 142 kb)
ESM Table 2
The effect of exposure to GDM in utero on childhood adiposity outcomes in multivariate linear regression analysis for non Hispanic white and Hispanic youthsa (PDF 52 kb)
Rights and permissions
About this article
Cite this article
Crume, T.L., Ogden, L., West, N.A. et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 54, 87–92 (2011). https://doi.org/10.1007/s00125-010-1925-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1925-3