Published online Aug 15, 2013. doi: 10.4239/wjd.v4.i4.101
Revised: June 27, 2013
Accepted: July 17, 2013
Published online: August 15, 2013
Osteoporosis has become a serious health problem throughout the world which is associated with an increased risk of bone fractures and mortality among the people of middle to old ages. Diabetes is also a major health problem among the people of all age ranges and the sufferers due to this abnormality increasing day by day. The aim of this review is to summarize the possible mechanisms through which diabetes may induce osteoporosis. Diabetes mellitus generally exerts its effect on different parts of the body including bone cells specially the osteoblast and osteoclast, muscles, retina of the eyes, adipose tissue, endocrine system specially parathyroid hormone (PTH) and estrogen, cytokines, nervous system and digestive system. Diabetes negatively regulates osteoblast differentiation and function while positively regulates osteoclast differentiation and function through the regulation of different intermediate factors and thereby decreases bone formation while increases bone resorption. Some factors such as diabetic neuropathy, reactive oxygen species, Vitamin D, PTH have their effects on muscle cells. Diabetes decreases the muscle strength through regulating these factors in various ways and ultimately increases the risk of fall that may cause bone fractures.
Core tip: The physical complications due to diabetes mellitus are not limited since there have been going research to elucidate the relation of other diseases with diabetes mellitus (DM). Osteoporosis is one of the complicated diseases of human that may be linked with DM through different networks in the body. In this review a precise relationship has been made between DM and osteoporosis through a broad range of biophysical pathways.
- Citation: Roy B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes 2013; 4(4): 101-113
- URL: https://www.wjgnet.com/1948-9358/full/v4/i4/101.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i4.101
Osteoporosis (OP) has become an alarming health problem through the entire world and about 200 million people in the world are under the threat of this deleterious health problem[1]. Although OP is often described as a silent disease because it is typically asymptomatic until a fracture occurs, the disease negatively and significantly impacts morbidity and mortality as it can lead to severe pain, deformity, disability, and death[2]. The signs of OP are deterioration of the microstructure of bone specifically at trabecular sites including vertebrae, ribs and hips, culmination in fragility fractures, pain and disability[2,3]. The occurrence of OP is prevalent among the aging women than the aging men although corticosteroid treatment, intake of excessive alcohol, cigarette smoking, low calcium intake and hypogonadism may be the secondary cause[1,2].
Like osteoporosis, diabetes mellitus is a pandemic and a chronic metabolic disorder with substantial morbidity and mortality, characterized by the presence of high blood glucose[2,4,5]. According to the report (September 2012) of the World Health Organization (WHO) about 374 million people in the world are under the threat of this deleterious health problem[6]. Under chronic condition DM adversely affects the different parts of the body including bone, nerve, muscles, retina of the eyes, cardiovascular system and nephron of kidney[4]. The effects of DM on bone cell are very complex and several investigations have been conducted to explore the exact mechanisms through which DM induces osteoporosis and bone fractures and all the investigations have come to the end with few findings[6]. The exact mechanism of diabetes mellitus (DM) induced osteoporosis is almost unknown but it is plausible that, patients with DM have increased rate of osteoporosis and bone fractures[3,7-10]. Hyperglycemia may induce osteoporosis and bone fractures through exerting its effects on bone cells and muscle cells through different possible pathways. This review has explained the possible molecular mechanisms through which DM may induce osteoporosis and bone fractures.
The bone mainly comprise of three basic types of cells osteoblast, osteocyte and osteoclast[11]. Osteoblasts commonly called bone-forming cells which derived from the osteoblast progenitor cells, participate in mineralization and are unable to multiply[11]. Osteocytes are mature osteoblast which no longer secretes matrix, participates in nutrient/waste exchange via blood and unable to divide. Osteoclasts are cells that derive from the macrophage-monocyte cell lineage and participate in bone resorption[1,11].
Osteoblast originates from the mesodermal progenitor cell and among the three basic types of bone cells it plays a crucial role in bone formation. Binding of different types of growth factors and hormones including bone morphogenetic protein (BMP), Wnt, transforming growth factor-β (TGF-β), parathyroid hormone (PTH), platelet derived growth factors (PDGFs), fibroblast growth factors (FGF) with their receptors expressed on the cell surface of mesodermal progenitor cells (also known as mesenchymal stem cells) induce the activation of different types of transcription factors responsible for osteoblast differentiation, maturation and survival[1,12].
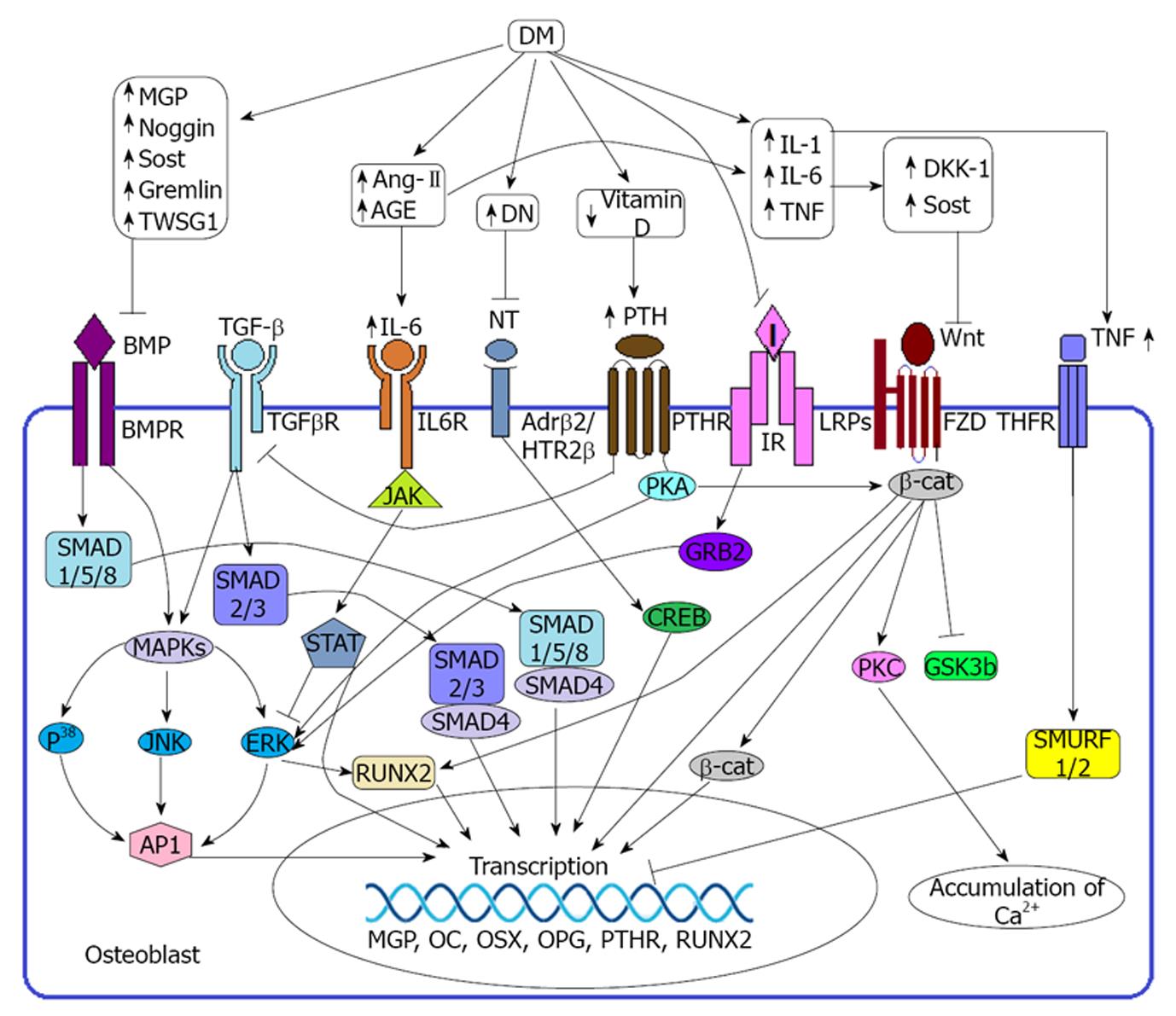
BMPs are the members of TGF-β superfamily and known to be a potent inducer of osteoblast formation and thereby increase collagen synthesis and decrease collagenase-3 production[1,13]. There are several types of BMP proteins and among them BMP-2, BMP-4, BMP-5, BMP-6 and BMP-7 have strong capacity in osteogenesis[14]. BMP-2 and BMP-6 induce osteoblast formation and chondrocyte proliferation[14,15]. BMP-4 could participate in endochondralossification[16,17] and BMP-7 induces the expression of markers including ALP activity and accelerated calcium mineralization which are required for osteoblast differentiation[14]. But BMP-3 has adverse effect on osteoblastogenesis[14]. BMP signaling has been identified as the major signaling molecules in the pre osteoblast because the binding of BMPs to its receptors (BMPRs) induce phosphorylation of SMADs proteins specially SMAD-1, SMAD-5 and SMAD-8 (Figure 1). SMADs then in turn directly activate the SMAD binding element (SBE) through the SMAD depended pathway and thereby induces the transcription of corresponding genes. On the non SMAD depended pathway BMPRs directly activate MAPK and then in turn activate the particular genes through inducing runt related transcription factor 2 (RUNX2) or activator protein 1 (AP-1)[14,18,19].
Wnt is the member of highly conserved secreted glycoprotein family, rich in cystein residue and are divided into two classes: canonical Wnts (wnt1, wnt3a) and non-canonical Wnts (wnt5a). Binding of canonical Wnts with frizzled (FZD) and LDL receptor related proteins (LRPs) promotes: the phosphorylation and inactivation of glycogen synthase kinase 3 beta (GSK3b), prevents the degradation of β-catenin (β-cat) as well as subsequent translocation of β-cat in the nucleus for binding with the target genes (Figure 1). Binding of non-canonical wnts with FZD receptor promote the activation of heterotrimeric G proteins in order to enhance the deposition of intracellular calcium ion (Ca2+) through protein kinase C (PKC) mediated pathway or induce the formation of the cytoskeleton via Rho/c-Jun N-terminal kinase dependent mechanism[18,19].
TGF-β signaling is important for the regulation, proliferation and commitment to the osteoblastic lineage of MSC. Binding of TGF-β with its receptor TGFβR regulates the expression of target genes through two possible pathways: canonical and non-canonical. In the canonical or smad dependent pathway activated TGFβR promotes the phosphorylation of R-SMADs (SMAD-2, 3) and thereby activate the target genes through SMAD-4 m = ediated signal transduction (Figure 1). In the non-canonical or non smad dependent pathway activated TGFβR promotes the expression of responsive genes through MAPK, P38, ERK mediated signal transduction pathway[12,14].
Immunohistochemical analysis revealed that the periosteum and bone are linked with the sympathetic, sensory and the glutaminergic nervous system specifically the growth plate and the metaphysic of long bones are more exposed to the neural network. Close contact of the nervous system with the bone cells, strongly implying a physiological role of neural signal on bone health[20,21]. In addition, osteoblast has been reported to express β-2 adrenergic receptors (β2AR) and 5-hydroxytryptamine receptor (5HTR) for several neurotransmitters including serotonin and norepinephrine[21,22]. An in vivo experiment showed that 5HTR 2β facilitate osteoblast recruitment and proliferation and the absence of this receptor leads to osteopenia[22]. Binding of neurotransmitter with particular receptors activates the transcription factor CREB and ultimately induces the gene for osteoblast proliferation through AP1 activation[18](Figure 1).
Elevated secretion of PTH has been reported to sequester osteoblast differentiation and activation. Attachment of PTH with its receptor PTHR activates protein kinase A (PKA) and extracellular signal regulated kinase (ERK) and ultimately induces the expression of matrix gla protein (MGP) on osteoblast which is a potent inhibitor of BMP signaling[1,14]. PTH binding also drives internalization of PTHR-TGFβR complex, which attenuates TGF-β signaling in bone development[14].
Several extracellular, intracellular and transcriptional BMP inhibitors such as matrix gla protein (MGP), Noggin, dickkopf-related protein 1 (DKK-1), Sclerostin, Gremlin, Ski, Smurf-1, Smurf-2, twisted gastrulation (Twsg1), Interleukin 6 (IL-6) and TNFs have been identified in the down regulation of BMP and TGF-β signaling pathways and ultimately suppress osteoblast function[1,13,14,23,24]. MGP is the member of mineral binding γ-carboxyglutamic acid containing protein family that directly and indirectly sequesters mineralization of bone cells. In the direct effect it acts as a part of a complex with α-2-HS glycoprotein and in the indirect effect it inhibits the binding of BMP-2 with its receptor expressed on the osteoblast precursors[1].
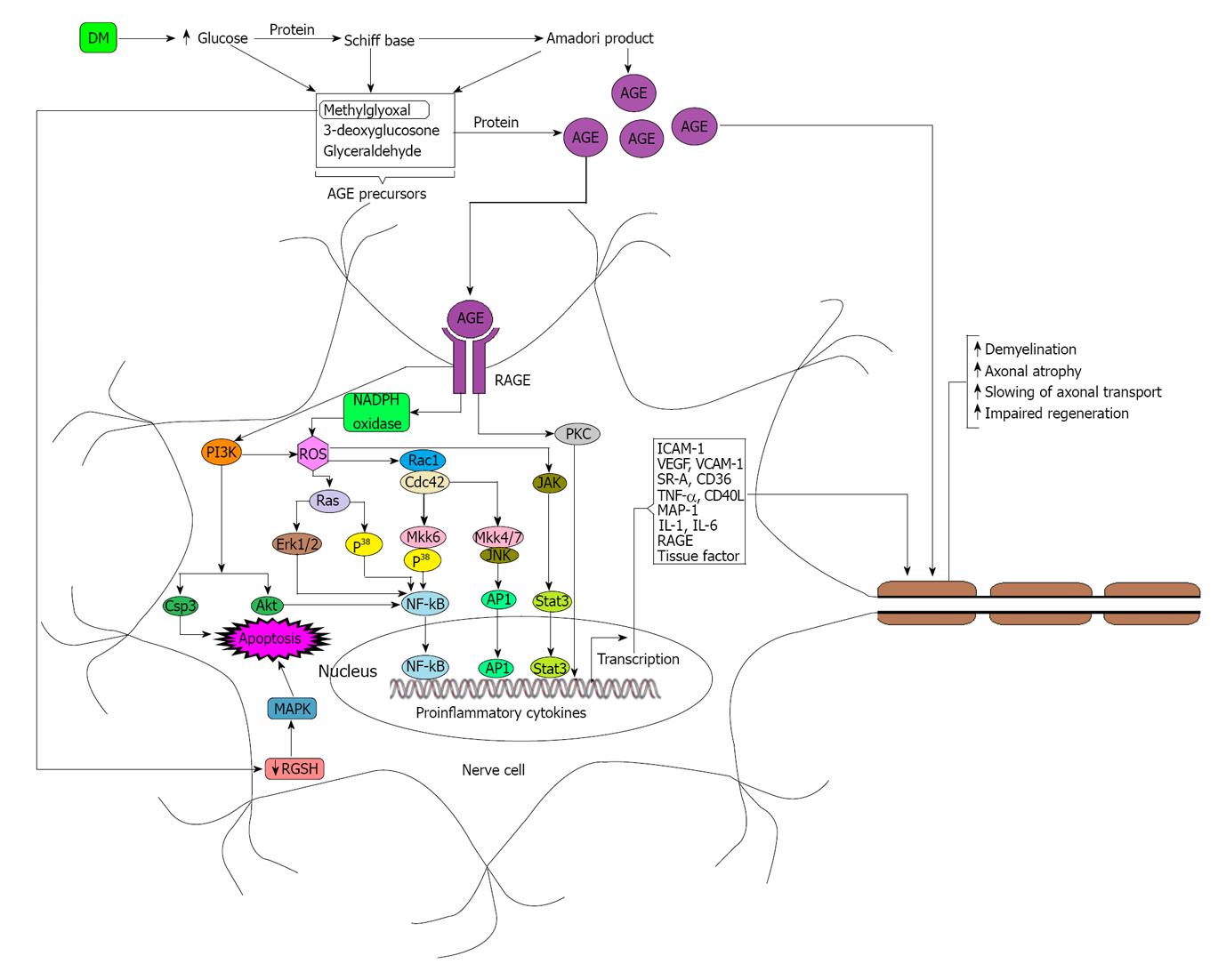
Diabetes mellitus (DM) not only induces the overexpression of DKK-1[25,26] Sclerostin[27,28] Gremlin[29,30] PTH[31] angiotensin II (Ang-II)[32] IL-6[33] and TNFs[33-35] but also sequesters the over expression of Vitamin D and neurotransmitters required for the normal growth of osteoblast. DM induced diabetic neuropathy is the commonest complication of non-traumatic lower limb amputations in diabetic patients. Although the exact pathogenesis of diabetic neuropathy remains unclear, there are emerging data from in-vitro and in-vivo clinical studies suggesting that hyperglycemia induced formation of advanced glycation end products (AGEs) may play a key role in the pathogenesis of diabetic neuropathy[36,37]. Under hyperglycemic conditions, concentrations of methylglyoxal, 3-deoxyglucosone and glyceraldehyde increase rapidly due to the increased breakdown of glucose. Elevated levels of methylglyoxal, 3-deoxyglucosone and glyceraldehyde lead to the formation of advance AGEs which in turn modify nerve cell components as well as signal through the receptor for advance glycation end product (RAGE) expressed on the nerve cells in order to produce different types of cytokines which may have roles on nerve damage[36-38]. AGEs have deleterious effect on nerve cells because they modify neuronal proteins including tubulin, neurofilament, laminin and actin through glycation and thereby sequester the nerve function (Figure 2)[36,37].
Beyond the damage of peripheral nerve cells on osteoblast through diabetic neuropathy, DM induced AGEs and angiotensin-II also upregulate the expression of IL-6 that regulates osteoblastic genes required for their survival, differentiation and function[32,39,40](Figure 1).
Reduced vitamin D levels in the body have been identified as a potential risk factor of osteoporosis and bone fractures. Deficiency of Vitamin D in the serum sequesters the intestine to absorb Ca2+ from diet and thereby signals the parathyroid gland to secrete elevated levels of PTH. Hyper secretion of PTH induces bone resorption and inhibit osteoblastogenesis in order to maintain the optimal level of calcium and phosphorus in the blood required for metabolic process and neuromuscular functions[41,42]. Through binding of PTH with its receptor PTH-1 expressed on osteoblast triggers intracellular signaling molecules such as PKA, mitogen activated protein kinase A (MAPK), cyclic AMP-responsive element binding protein, AP1 and RUNX2 and thereby induce the expression of the MGP responsive element[1](Figure 1).
Bone marrow derived endothelial progenitor cells (EPCs) may have roles in angiogenesis during bone healing[3,43]. DM down regulates the expression of EPCs through different mechanisms and hereby decreases the rate of angiogenesis required for bone formation in the fracture sites[3,44,45]. Mesenchymal stem cells (MSC) derived from bone marrow act as a precursor of osteoblast formation[46-48]. Several labs based trials have come to the decision that, DM is responsible for the upregulation of peroxisome proliferator-activated receptor-γ (PPAR-γ), adipocyte fatty acid binding protein (aP2), TNF-α and consequently decrease the availability of MSC for osteoblast formation but increase the availability of MSC for adipocyte formation[3,4,34,35,48,49]. So it is intuitive that, in addition to direct interference with osteoblast formation DM also responsible for the deposition of lipid in the bone marrow and thereby leading to the expansion of marrow cavity as well as decreases the rate of blood flows to the bone which is required for the transfer of nutrients[3,5]. The transformation of osteoblast to adipocyte makes the reduction of osteoblast number available for bone formation[3,50]. Advanced glycation end products (AGEs) have been identified as a biomarker for the increased risk of fractures because it decreases the synthesis of type I collagen and thereby decreases the bone strength. It is now well researched that DM is responsible for the over expression of AGE and have roles in bone rigidity[51-53].
Several experimental studies implicated that, insulin has an anabolic effect on osteoblast development and it is intuitive that, insulin may exert its effect on osteoblast through IR-GRB2-ERK mediated pathway[4,54](Figure 1). Beyond the synthesis of insulin pancreatic β cells also produce other osteoporotic factors including amylin and preptin. Amylin induces bone formation and sequesters bone resorption, preptin induces osteoblast differentiation and mineralization as well as reducing the apoptosis of osteoblast[4]. Osteocalcin is a peptide which positively regulates osteogenesis. DM limits the production of osteocalcin through the negative regulation of osteoblast by decreased synthesis of insulin, amylin and preptin. Testosterone is also an important factor of osteogenesis and it is obvious that, limited production of osteocalcin reduces the production of testosterone from the testes[4].
Osteoclasts are cells that derived from the monocyte-macrophage cell lineage and strongly participate in osteoclastogenesis. It is well documented that different types of mediators such as nuclear factor κ-B (NF-κB), receptor activator for nuclear factor κ-B ligand (RANKL), osteopontin (OPN), parathyroid hormone (PTH), macrophage colony stimulating factor (M-CSF), and angiotensin-II (AT-II) have prominent roles to induce osteoclastogenesis[1,13].
In general osteoclast exerts its effects in osteoclastogenesis through three possible pathways (1) RANKL mediated; (2) M-CSF mediated; and (3) immunoreceptor tyrosine-based activation motifs (ITAMs). But in inflammatory condition osteoclastogenesis may take place through other pathways like MCP mediated, TNF mediated and IL-6 mediated[12,55].
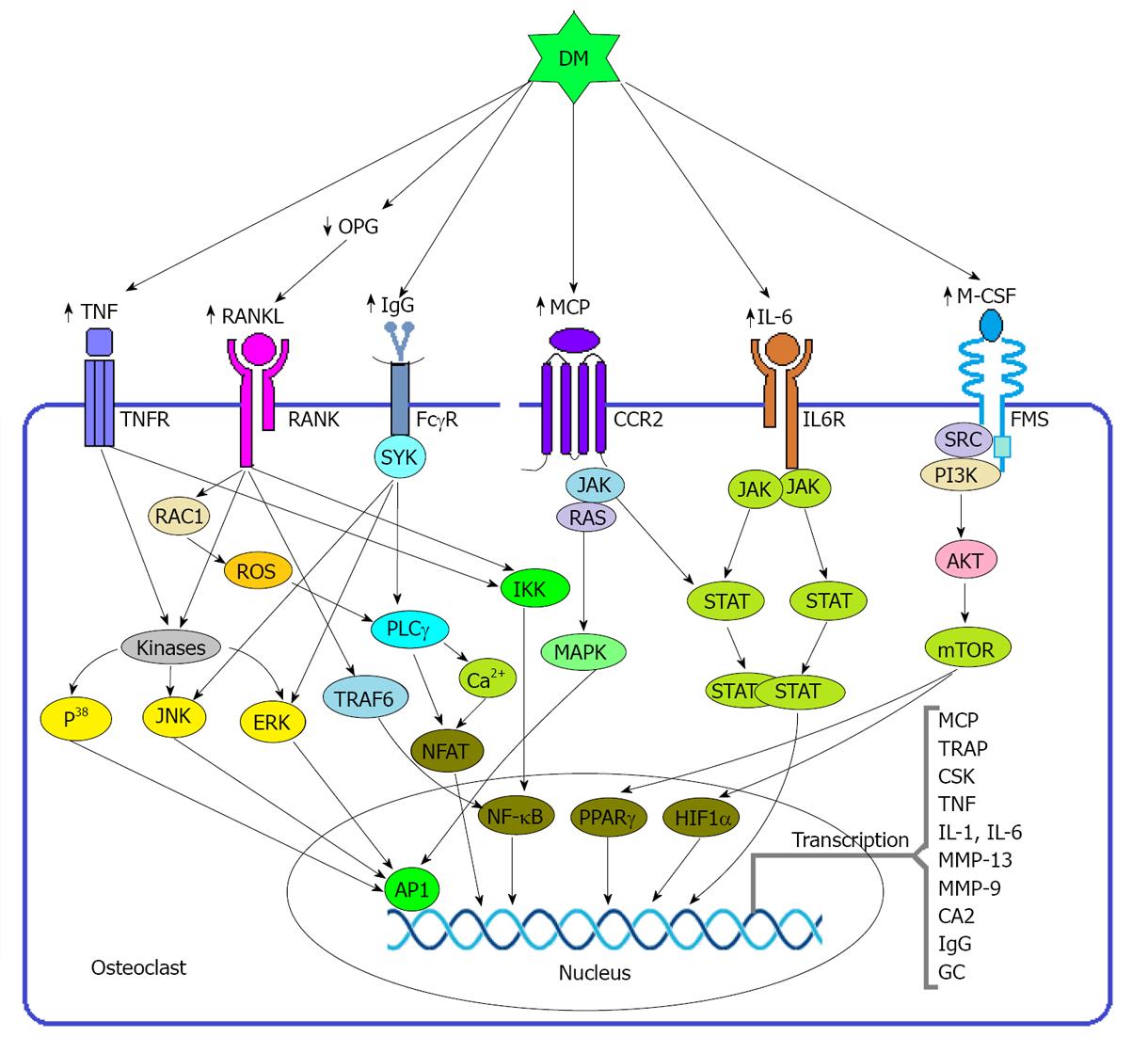
RANKL is a key factor derived from osteoblast and stromal cells, binds with the receptor expressed on the cell surface of monocyte-macrophage cell lineage and thereby triggers the differentiation of pre osteoclast to osteoclast through activating NF-κB and NFATc1[56]. RANKL inhibits the apoptosis of osteoclast through inducing the anti-apoptotic enzyme protein kinase B (PKB) (Figure 3). RANKL also responsible for the production of reactive oxygen species (ROS) including free radicals, oxygen ions and peroxides which are potent inducer of osteoclastogenesis[1,12,56-59].
Binding of RANKL with its receptor RANK activates signal transduction pathways involving the adaptor protein TNF receptor-associated factor 6. Subsequently, several kinases such as p38 MAPK and JUN N-terminal kinase 1 are activated, which in turn induce the transcription via the various hetero and homodimers of the AP1 family of proteins including FOS, FOSB, FOS-related antigen 1 (FRA1), FRA2, JUN, JUNB and JUND (Figure 3). AP1 regulates the differentiation, proliferation and apoptosis, of various cell types[12].
RANKL is necessary for osteoclastogenesis but an experiment conducted on mouse model showed that, M-CSF acts as a positive catalyst in RANKL activation because the addition of M-CSF requires less time to do a particular resorption process than the RANKL alone[60]. Osteoprotegerin (OPG) is a prominent factor for osteoclast activation because the affinity of OPG for RANKL prevents the binding of RANKL with its receptor RANK and thereby decrease the RANKL-RANK mediated pathway of octeoclast multiplication, survival and bone resorption[1].
According to the immunoreceptor tyrosine-based activation motifs (ITAMs), binding of immune complex like immunoglobulin G (IgG) with its receptor FcγR activates spleen tyrosine kinase (SYK), which in turn induces NFATC1 through the activation of phospholipase Cγ (PLCγ) (Figure 3). NFATC1 is an important transcription factor that transcribes the genes that encode calcitonin receptor, tartrate-resistant acid phosphatase, matrix metalloproteinase 13 and cathepsin K. All these factors enable the acidification and degradation of the bony matrix[12]. DM is thought to be a potent inducer of IgG because an experiment conducted on mouse model showed that non-obese diabetic mice spontaneously produce natural IgG autoantibodies[61].
Beyond the roles of RANKL and M-CSF in osteoclastogenesis, on the state of hyperglycemia a group of proinflammatory cytokines is activated including TNF, IL-1 and IL-6 and these cytokines have profound effects on the differentiation and activation of osteoclast[12,62-64]. Although osteoclast differentiation and activation is primarily dependent on the presence of M-CSF and RANKL, osteoclastogenesis is enhanced in the presence of TNF, IL-1 or IL-6. This is partly a consequence of the induction of RANKL in target cells, but these pro-inflammatory cytokines also responsible for the differentiation and activation of osteoclasts from the preosteoclast. In addition, under normal concentrations of RANKL, TNF can induce the differentiation of monocytes and macrophages to preosteoclasts. The osteoclastogenic activity of TNF is mediated by p55 TNF receptor and may be partly counteracted by the activation of the p75 TNF receptor[12].
IL-6 is thought to be the most abundant and effective cytokines in blood because: (1) the concentration of IL-6 and IL-6 receptor (IL-6R) is higher than the other cytokines; (2) IL-6 mediates the production of other cytokines related to osteoclastogenesis like glucocorticoid (Figure 3); and (3) Estrogen deficiency exerts its effects in osteoclastogenesis via IL-6 mediated pathway as well as IL-6 is a potent inducer of IgG production[12,61,65].
DM not only induces the overexpression of RANKL[3,66] M-CSF[3,66] NF-κB[67] and OPN[34,68] but also stimuli the over expression of several proinflammatory stimulus such as IL-6, MCP, IgG and TNFs which are so important for the maturation and activation of osteoclast. The DM may induce monocyte to secrete IL-6 through ROS, PKC, MAPK, and NF-κB mediated pathways[69,70].
Estrogen deficiency stimulates osteoclast formation not only by decreasing the OPG production but also by increasing the production of TNF-α, RANKL and osteoclast precursors through stimulating the T cells[71,72]. There is striking evidence on behalf of this regard that, estrogen levels are significantly lower in DM patients[73].Adiponectin is another factor secreted by the adipose tissue and there has been increasing evidence suggest that, adiponectin stimulates the differentiation and mineralization of osteoblast but directly inhibits osteoclast activity and bone resorption[74]. Some in situ studies have shown that adiponectin percentage is lower in individuals with DM than the individuals without DM[75].
Intracellular ROS mediated oxidative stress plays a crucial role in bone health because ROS promotes RANKL mediated osteoclast differentiation and function. Patients with type 2 DM have shown elevated level of mitochondrial ROS and thus supporting the point that, DM may have another role in ROS mediated osteolysis and bone fractures[76,77]. As mentioned before, diabetic neuropathy is a cause of increased production of IL-6, TNF and some other factors, so it is intuitive that, diabetic neuropathy may have a positive role in osteoclast functioning[12,38,64].
Muscle atrophy is a physiological condition which associated with the depression of protein synthesis as well as an increase in protein degradation[78]. There are some other evidences showed that, DM is associated with diabetic neuropathy mediated muscle atrophy or directly triggers muscle atrophy through TNF-α, NF-κB mediated pathway and thereby induces muscle weakness[68,79-81]. Weakness of the muscle is a risk factor of bone fractures because an individual with weak muscles is more likely to fall down than a normal individual. In addition to muscle weakness, diabetic polyneuropathy also induces bone resorption through osteolysis[82,83].
DM is directly associated with muscle atrophy through an increased activity of the ubiquitin proteosome system (UPS) although other pathways may involve in this process[78]. There are several inducers of UPS including glucose[33] TNF-α[84,85] Ang-II[86] IL-6, Glucocorticoid (GC)[85] and most of them exert their effects on myogenesis responsive gene through NF-κB mediated pathway[68,78].
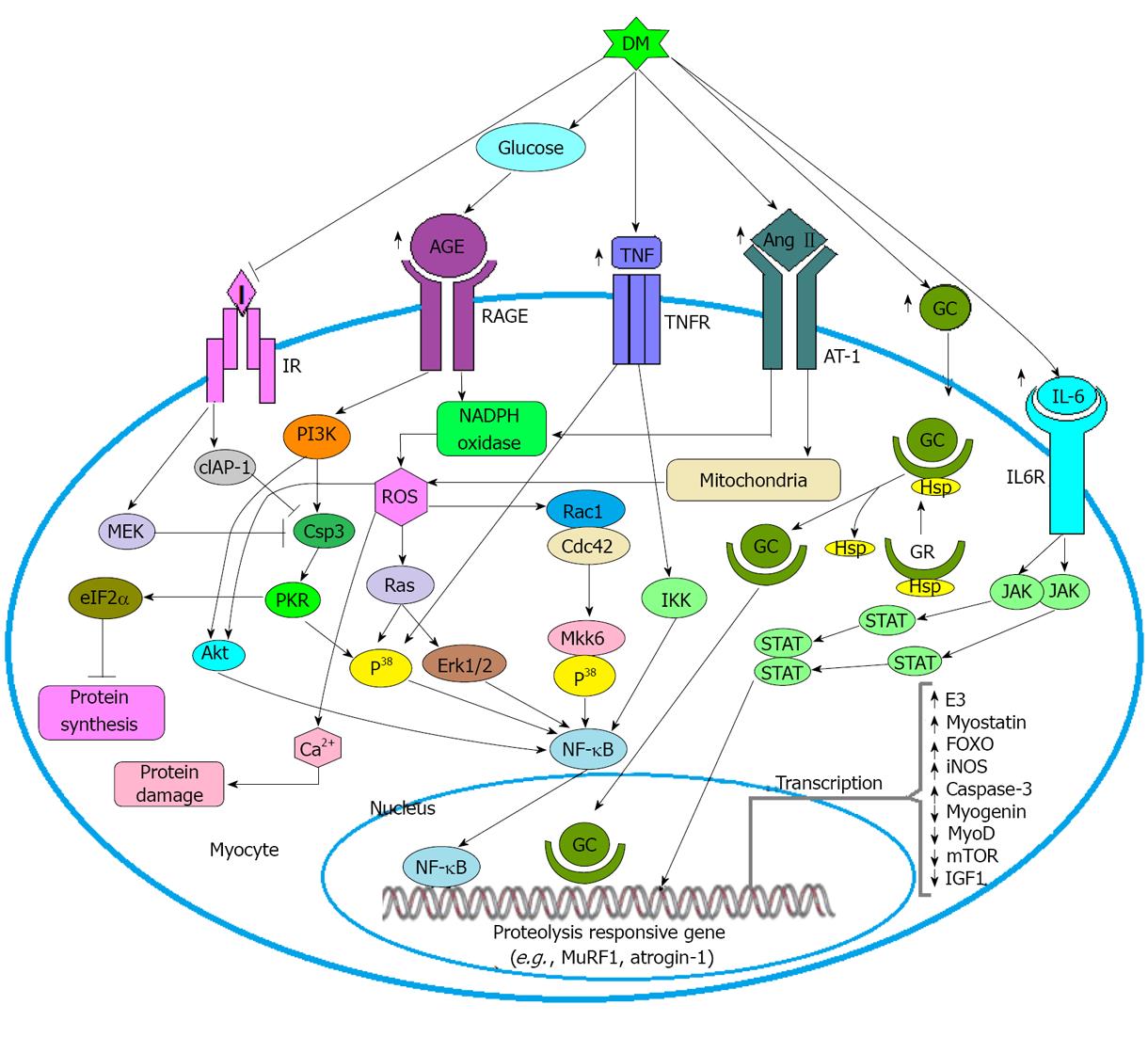
High extracellular glucose concentrations is a potential precursor of AGE formation and several evidences have shown that, AGE may induce the formation of ROS through NADPH oxidase and PI3K/Akt mediated pathway and ultimately activates the transcription factor NF-κB[37,38,78]. AGE may induce PKR through caspase-3 mediated pathway and activated PKR then in turn induces NF-κB through P38 MAPK mediated pathway as well as activates eIF2α which would depress protein synthesis by decreasing translational efficiency[78](Figure 4).
Several studies have implicated that TNF-α is a prominent cytokine in cachexia induced muscle atrophy[84] as well as a potent inducer of insulin resistance[87].Binding of TNF-α with its receptor expressed on myocyte activates nuclear transcription factor NF-κB through P38MAPK or IKK mediated pathway and activated NF-κB then in turn induces the transcription of inducible nitric oxide synthase (iNOS) as well as transcribes the gene MuRF-1 responsible for muscle wasting[84](Figure 4).
Ang-II which is the major peptide of the renin-angiotensin system has been implicated as a modulator of muscle wasting[88]. Ang-II exerts its effect on muscle atrophy not only through the generation of ROS but also through the activation of IL-6 and Glucocorticoid as well as through disrupting insulin signaling in muscle cells. It is experimentally determined that, ROS has a significant role in the reduction of muscle strength[89,90]. There are two sources of Ang-II induced ROS production (1) NADPH oxidase; and (2) Mitochondria, but NADPH oxidase is thought to be prominent between the two sources. ROS may contribute to muscle wasting activity through three mechanisms (1) by increasing the absorption of Ca2+ in order to activate calcium-activated proteases; (2) by stimulating the UPS through activating caspase-3; and (3) by up-regulating atrogin-1 and MuRF-1 in muscle to activate the proteasome system through transcribing E3 ligases[86](Figure 4).
Ang-II induced glucocorticoid (GC) plays an important role in muscle wasting because several in-vivo and in-vitro studies have shown that, addition of different types of GC antagonist of experimental model reduce the rate of muscle wasting[86,91,92]. GC exerts its effect on muscle through two ways (1) through sequestering the anabolic action, and (2) through inducing the catabolic action[91]. On behalf of the anti-anabolic action firstly, GC inhibits the transport of amino acids into the muscle and thereby limits the protein synthesis[91]. Secondly, GC sequesters the stimulatory effects of insulin and insulin like growth factor 1 (IGF-1)[91,92]. Thirdly, GC negatively regulates the synthesis of MyoD, an important transcription factor that regulates the differentiation and development of muscle cells as well as required for regeneration and self-renewal of skeletal muscle cells[92]. Fourthly, mechanistic target of rapamycin (mTOR) is a kinase protein which regulates the translation of muscle protein. GC inhibits the activity of mTOR through enhancing the transcription of REDD1, a repressor of mTOR function[91]. Finally, GC inhibits myogenesis through the downregulation of myogenin, an important a transcription factor required for differentiation of satellite cells into myofibrils[91,92]. On behalf of the catabolic activity firstly, GC stimulates the synthesis of several components (e.g., E3) required for UPS through the upregulation of the respective genes including MuRF-1 and atrogin-1[91,92]. Secondly, GC induces the overexpression of myostatin a growth regulator which inhibits the development of muscle mass through downregulating the proliferation and differentiation of satellite cells[91,93,94]. Thirdly, GC induces the breakdown of myofibrillar protein through the upregulation of caspase-3[89]. Finally, Forkhead Box O-1 (FOXO-1) is a transcription factor that induces UPS through the upregulation of genes including atrogin-1/MAFbx and MuRF1. Several lab based experiments have come to the decision that, GC induces the production of FOXO-1 through stimulating the respective genes[91,92](Figure 4).
IL-6 is a proinflammatory cytokine which has been implicated as a potential factor of muscle atrophy[86,95,96]. Ang-II induced IL-6 upregulates the transcription of serum amyloid A (SAA) and both of the factors (IL-6 and SAA) act synergistically to trigger muscle atrophy[93]. An in-vitro study has shown that, IL-6 exerts its effect on muscle wasting through JAK/STAT mediated pathway[97](Figure 4).
Insulin deficiency (ID) and insulin resistance (IR) are the hallmark of type-1 and type-2 DM respectively. IR has been implicated as a potential inducer of overall protein degradation as well as caspase-3 mediated actin cleavage. Elevated level of intracellular insulin inhibits caspase-3 protein through MEK and cIAP-1 mediated pathway but during IR or ID condition insufficiency of insulin cannot exert its inhibitory effect on caspase-3[98](Figure 4).
DM induced diabetic retinopathy may be another risk factor of bone fractures because diabetic retinopathy is a leading cause of vision loss and blindness and consequently augments the rate of stumble mediated bone fractures[99]. Abnormal movement caused by polyneuropathy and heart failure caused by diabetic cardiovascular complications also promotes the rate of fall[4,5].
Beyond the role of vitamin D in osteolysis, it is intuitive that, vitamin D exerts a range of effects in skeletal muscle cells. Muscle activity specially the power stroke is a Ca2+ depended process and due to the lack of Ca2+ the system will be shut down. An inadequacy of vitamin D can turn down the availability of calcium and phosphorus and thereby postpones the activity of muscles[100]. Some in vitro and in vivo trial have shown that, vitamin D levels are significantly lower in patients with DM[101,102].
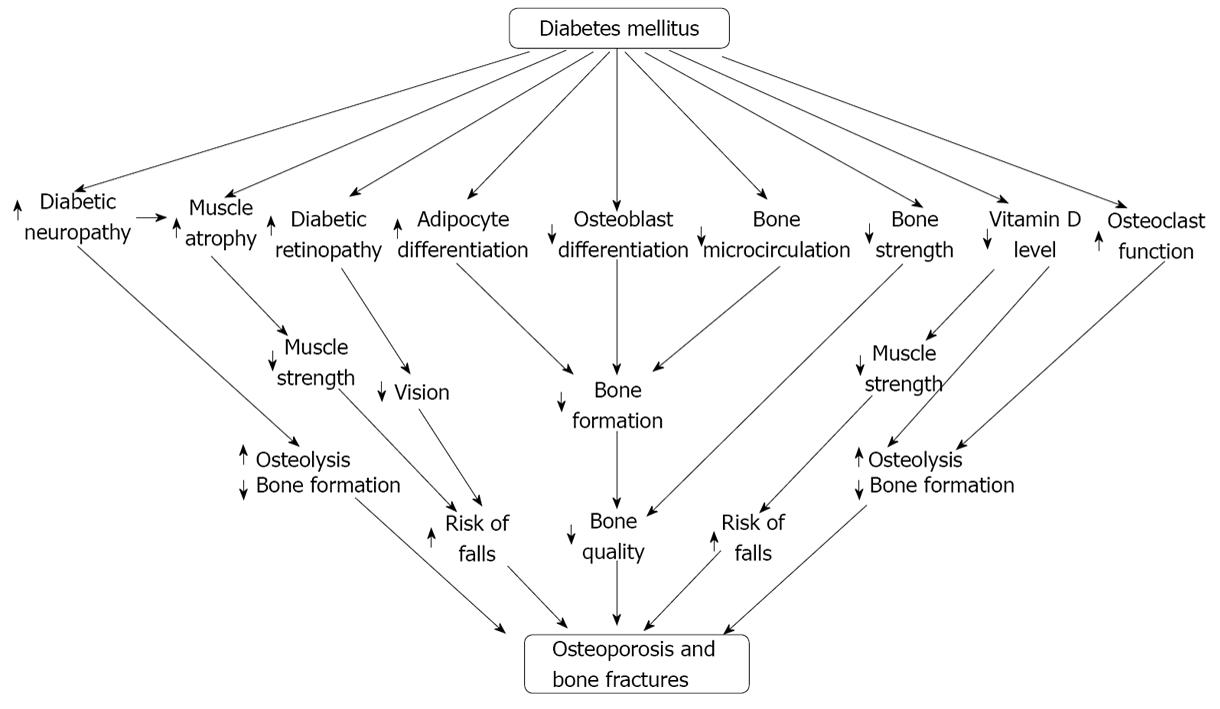
Diabetes mellitus may be an obvious cause of osteoporosis and bone fractures due to its broad range of effects on different mediators of the human body. It mainly regulates the bone cells (specifically osteoblast and osteoclast) and the muscles to exert its effects to facilitate osteoporosis as well as reduction of muscle strength[3,98](Figure 5). DM negatively regulates the normal functioning of osteoblast but positively regulates the osteoclast functioning in order to facilitate the process of osteoporosis[1,98]. DM reduces the availability of MSC to produce osteoblast but simultaneously increases the availability of MSC for adipocyte formation[1]. Due to the continual differentiation and deposition of adipocytes into the bone marrow increase the bone marrow cavity to make the bone fragile as well as decrease the bone microcirculation[1,99]. The limitation of osteoblast functioning, over production of adipocytes and fluent functioning of osteoclasts all these effects negatively regulate the bone formation but positively regulate the bone resorption and ultimately cause osteoporosis. DM induced diabetic neuropathy acts as a prominent factor in osteoporosis and muscle atrophy. Diabetic neuropathy inhibits bone formation through sequestering osteoblast formation and function but facilitates osteolysis through inducing the osteoclast generation and function as well as reducing muscle strength through inducing the expression of different cytokines and ROS[75,98]. DM directly causes muscle atrophy through different mediators or indirectly causes muscle atrophy through diabetic neuropathy. DM induced diabetic retinopathy is another risk factor that may cause bone fractures through reducing eye sight[98]. Reduced muscle strength as well as reduced eye sight may cause elevated rate of fall down or stumble that may cause bone fractures. Vitamin D is an essential factor of bone and muscle activities because deficiency of vitamin D stimulates the production of PTH which is a negative regulator of osteoblast functioning but a positive regulator of osteoclast functioning which then in turn reduce bone formation and increase bone resorption respectively[53,54]. DM induced Vitamin D deficiency also causes the reduction of muscle strength because it lowers the rate of Ca2+ absorption by the intestine and thereby reduces the activity of muscle which may be a risk factor of bone fractures through increasing the rate of fall[55,98].
Diabetes mellitus exerts its diabolical effects on bone, neuron and muscle cells through a broad spectrum of mechanisms. It declines the production of various stimuli required for normal homeostasis of the above cells and accelerates the synthesis of several cytokines and other factors which may directly destroy the target cells or indirectly antagonize the signaling pathways of the stimulus. As human body is a network of different pathways so any imbalance on any part of the pathway may tends the body vulnerable to different threats. DM is the potent source of excess glucose in the blood which is the principal key to create a lot of abnormalities in the body including Osteoporosis and bone fractures, cardiovascular disease, diabetic nephropathy, diabetic neuropathy, Diabetic retinopathy and muscle atrophy. Other fatal diseases like HIV and cancer may be linked with hyperglycemia and several investigations have been running to elucidate the mystery of DM induced mechanism. Although several drugs are available to treat osteoporosis, regular physical exercise would be a better way to get rid of from this type of life threatening disease.
P- Reviewers Scuteri A, Wong WTJ S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Lampropoulos CE, Papaioannou I, D’Cruz DP. Osteoporosis--a risk factor for cardiovascular disease? Nat Rev Rheumatol. 2012;8:587-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Chen HL, Deng LL, Li JF. Prevalence of Osteoporosis and Its Associated Factors among Older Men with Type 2 Diabetes. Int J Endocrinol. 2013;2013:285729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Wongdee K, Charoenphandhu N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J Diabetes. 2011;2:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone--osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence. 2012;6:435-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Sealand R, Razavi C, Adler RA. Diabetes mellitus and osteoporosis. Curr Diab Rep. 2013;13:411-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Won HY, Lee JA, Park ZS, Song JS, Kim HY, Jang SM, Yoo SE, Rhee Y, Hwang ES, Bae MA. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS One. 2011;6:e18168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev. 2010;26:622-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Paula FJ, Rosen CJ. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq Bras Endocrinol Metabol. 2010;54:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Räkel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008;34:193-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 Suppl 3:S131-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1309] [Cited by in F6Publishing: 1064] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 12. | Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 527] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 13. | Biver E, Hardouin P, Caverzasio J. The “bone morphogenic proteins” pathways in bone and joint diseases: translational perspectives from physiopathology to therapeutic targets. Cytokine Growth Factor Rev. 2013;24:69-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1052] [Cited by in F6Publishing: 1165] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 15. | Mizrahi O, Sheyn D, Tawackoli W, Kallai I, Oh A, Su S, Da X, Zarrini P, Cook-Wiens G, Gazit D. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013;20:370-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Lin L, Fu X, Zhang X, Chen LX, Zhang JY, Yu CL, Ma KT, Zhou CY. Rat adipose-derived stromal cells expressing BMP4 induce ectopic bone formation in vitro and in vivo. Acta Pharmacol Sin. 2006;27:1608-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, Gallick GE, Maity SN, Lin SH. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011;71:5194-5203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068-3092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 426] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 19. | Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens). 2007;6:279-294. [PubMed] [Cited in This Article: ] |
| 20. | He JY, Jiang LS, Dai LY. The roles of the sympathetic nervous system in osteoporotic diseases: A review of experimental and clinical studies. Ageing Res Rev. 2011;10:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. β2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology. 2011;152:1412-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay JM, de Vernejoul MC. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 2008;22:418-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | O’Brien CA. Control of RANKL gene expression. Bone. 2010;46:911-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Thomsen SB, Rathcke CN, Zerahn B, Vestergaard H. Increased levels of the calcification marker matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc Diabetol. 2010;9:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Hie M, Iitsuka N, Otsuka T, Tsukamoto I. Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of Wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int J Mol Med. 2011;28:455-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH, Wang FS. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol. 2010;21:124-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | García-Martin A, Rozas-Moreno P, Reyes-García R, Morales Santana S, Garcia-Fontana B, Garcia-Salcedo JA, and Manuel Munoz-Torres Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J ClinEndocrinolMetab. 2012;97:234-241. [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3744-3750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Kane R, Stevenson L, Godson C, Stitt AW, O’Brien C. Gremlin gene expression in bovine retinal pericytes exposed to elevated glucose. Br J Ophthalmol. 2005;89:1638-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Russo LM, del Re E, Brown D, Lin HY. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy: amelioration by soluble TGF-beta type II receptor. Diabetes. 2007;56:380-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Taheri E, Saedisomeolia A, Djalali M, QorbaniM , Civi MM. The relationship between serum 25-hydroxy vitamin D concentration and obesity in type 2 diabetic patients and healthy subjects. Journal of Diabetes & Metabolic Disorders. 2012;11:16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Modesti A, Bertolozzi I, Gamberi T, Marchetta M, Lumachi C, Coppo M, Moroni F, Toscano T, Lucchese G, Gensini GF. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes. 2005;54:394-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Kim HJ, Kim SH, Yun JM. Fisetin Inhibits Hyperglycemia-Induced Proinflammatory Cytokine Production by Epigenetic Mechanisms. Evidence-Based Complementary and Alternative Medicine. 2012;2012:1-10. [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Masoud G, GhorbaniP , Ardestani MS. Treatment with CL316, 243 Improves Insulin Resistance by Down Regulation of Tumor Necrosis Factor-α (TNF-α) Expression. Insight Biomedical Science. 2012;2:6-9. [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Gonzalez Y, Herrera MT, Soldevila G, Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K, Guzmán-Beltrán S, Sada E, Torres M. High glucose concentrations induce TNF-a production through the down-regulation of CD33 in primary human monocytes. BMC Immunology. 2012;13:19. [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res. 2012;159:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Vazzana N, Santilli F, Cuccurullo C, Davì G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Rasheed Z, Akhtar N, Haqqi TM. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford). 2011;50:838-851. [PubMed] [Cited in This Article: ] |
| 40. | Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension. 2012;59:136-144. [PubMed] [Cited in This Article: ] |
| 41. | Stojanovic OI, Lazovic M, Lazovic M, Vuceljic M. Association between atherosclerosis and osteoporosis, the role of vitamin D. Arch Med Sci. 2011;7:179-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Binkley N. Vitamin D and osteoporosis-related fracture. Arch Biochem Biophys. 2012;523:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Schmidt-Bleek K, Schell H, Lienau J, Schulz N, Hoff P, Pfaff M, Schmidt G, Martin C, Perka C, Buttgereit F. Initial immune reaction and angiogenesis in bone healing. J Tissue Eng Regen Med. 2012;Apr 11; [Epub ahead of print]. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 44. | Menegazzo L, Albiero M, Avogaro A, Fadini GP. Endothelial progenitor cells in diabetes mellitus. Biofactors. 2012;38:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Balestrieri ML, Servillo L, Esposito A, D’Onofrio N, Giovane A, Casale R, Barbieri M, Paolisso P, Rizzo MR, Paolisso G. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia. 2013;56:162-172. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 47. | Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, Richards CD, Chevalier S, Rédini F, Heymann D. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 48. | Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 49. | Bojin FM, Gruia AT, Cristea MI, Ordodi VL, Paunescu V, Mic FA. Adipocytes differentiated in vitro from rat mesenchymal stem cells lack essential free fatty acids compared to adult adipocytes. Stem Cells Dev. 2012;21:507-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Sheng HH, Zhang GG, Cheung WH, Chan CW, Wang YX, Lee KM, Wang HF, Leung KS, Qin LL. Elevated adipogenesis of marrow mesenchymal stem cells during early steroid-associated osteonecrosis development. J Orthop Surg Res. 2007;2:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Vashishth D. Advanced glycation end-products and bone fractures. IBMS BoneKEy. 2009;6:268–278. [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Kranstuber AL, Del Rio C, Biesiadecki BJ, Hamlin RL, Ottobre J, Gyorke S, Lacombe VA. Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front Physiol. 2012;3:292. [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 582] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 54. | Fowlkes JL, Bunn R C, Thrailkill KM. Contributions of the Insulin/Insulin-Like Growth Factor-1 Axis to Diabetic Osteopathy. J Diabetes Metab. 2011;1:S1-003. [PubMed] [Cited in This Article: ] |
| 55. | Roux S. New treatment targets in osteoporosis. Joint Bone Spine. 2010;77:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010;285:6913-6921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 57. | Jules J, Zhang P, Ashley JW, Wei S, Shi Z, Liu J, Michalek SM, Feng X. Molecular basis of requirement of receptor activator of nuclear factor κB signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem. 2012;287:15728-15738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | McManus S, Roux S. The adaptor protein p62/SQSTM1 in osteoclast signaling pathways. J Mol Signal. 2012;7:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Shanmugarajan S, Haycraft CJ, Reddy SV, Ries WL. NIP45 negatively regulates RANK ligand induced osteoclast differentiation. J Cell Biochem. 2012;113:1274-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Hodge JM, Collier FM, Pavlos NJ, Kirkland MA, Nicholson GC. M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS One. 2011;6:e21462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Francisco J. Quintana, Milena Pitashny and Irun R. Cohen; Experimental autoimmune myasthenia gravis in naive non-obese diabetic (NOD/LtJ) mice: susceptibility associated with natural IgG antibodies to the acetylcholine receptor. International Immunology. 2003;15:11-16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Gonzalez Y, Herrera MT, Soldevila G, Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K, Guzmán-Beltrán S, Sada E, Torres M High glucose concentrations induce TNF-α production through the down-regulation of CD33 in primary human monocytes. BMC Immunology. 2012;13:19. [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 63. | Kim HJ, Kim SH, Yun JM. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med. 2012;2012:639469. [PubMed] [Cited in This Article: ] |
| 64. | Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009;50:1920-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Maeda K, Mehta H, Drevets DA, Coggeshall KM. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood. 2010;115:4699-4706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Liu W, Xu GZ, Jiang CH, Tian J. Macrophage colony-stimulating factor and its receptor signaling augment glycated albumin-induced retinal microglial inflammation in vitro. BMC Cell Biol. 2011;12:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Frier BC, Noble EG, Locke M. Diabetes-induced atrophy is associated with a muscle-specific alteration in NF-kappaB activation and expression. Cell Stress Chaperones. 2008;13:287-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Yan X, Sano M, Lu L, Wang W, Zhang Q, Zhang R, Wang L, Chen Q, Fukuda K, Shen W. Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2010;9:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes. 2005;54:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 70. | Chen JS, Chen YH, Huang PH, Tsai HY, Chen YL, Lin SJ, Chen JW. Ginkgo biloba extract reduces high-glucose-induced endothelial adhesion by inhibiting the redox-dependent interleukin-6 pathways. Cardiovascular Diabetology. 2012;11:49. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 72. | D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone. 2008;43:92-100. [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Cushman TT, Kim N, Hoyt R, Traish AM. Estradiol ameliorates diabetes-induced changes in vaginal structure of db/db mouse model. J Sex Med. 2009;6:2467-2479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Kanazawa I. Adiponectin in metabolic bone disease. Curr Med Chem. 2012;19:5481-5492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Su SC, Pei D, Hsieh CH, Hsiao FC, Wu CZ, Hung YJ. Circulating pro-inflammatory cytokines and adiponectin in young men with type 2 diabetes. Acta Diabetol. 2011;48:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Kafle D, Singh N, Singh SK, Singh N, Bhargav V, Singh AK. Persistent hyperglycemia generating reactive oxygen species in renal cells, a probable cause of inflammation in type2 diabetic nephropathy subjects. Biomed Res (India). 2012;23:501-504. [Cited in This Article: ] |
| 77. | Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, Degasperi A, Dymott JA, Hamilton CA, Murphy MP, Delles C. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci. 2013;124:403-411. [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Russell ST, Rajani S, Dhadda RS, Tisdale MJ. Mechanism of induction of muscle protein loss by hyperglycaemia. Exp Cell Res. 2009;315:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Bokan V. Muscle weakness and other late complications of diabetic polyneuropathy. Acta Clin Croat. 2011;50:351-355. [PubMed] [Cited in This Article: ] |
| 80. | van Sloten TT, Savelberg HH, Duimel-Peeters IG, Meijer K, Henry RM, Stehouwer CD, Schaper NC. Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res ClinPract. 2011;91:32-39. [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol. 2011;96:179-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Sinacore DR, Hastings MK, Bohnert KL, Fielder FA, Villareal DT, Blair VP, Johnson JE. Inflammatory osteolysis in diabetic neuropathic (charcot) arthropathies of the foot. Phys Ther. 2008;88:1399-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Mabilleau G, Edmonds ME. Role of neuropathy on fracture healing in Charcot neuro-osteoarthropathy. J Musculoskelet Neuronal Interact. 2010;10:84-91. [PubMed] [Cited in This Article: ] |
| 84. | Hall DT, Ma JF, Marco SD, Gallouzi IE. Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging (Albany NY). 2011;3:702-715. [PubMed] [Cited in This Article: ] |
| 85. | Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834-C843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 86. | Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 87. | Podbregar M, Lainscak M, Prelovsek O, Mars T. Cytokine response of cultured skeletal muscle cells stimulated with proinflammatory factors depends on differentiation stage. ScientificWorldJournal. 2013;2013:617170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Cabello-Verrugio C, Córdova G, Salas JD. Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci. 2012;13:560-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Smietana MJ, Arruda EM, Faulkner JA, Brooks SV, Larkin LM. Reactive oxygen species on bone mineral density and mechanics in Cu,Zn superoxide dismutase (Sod1) knockout mice. Biochem Biophys Res Commun. 2010;403:149-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, Avadhani NG. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010;1192:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 91. | Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 92. | Hasselgren PO, Alamdari N, Aversa Z, Gonnella P, Smith IJ, Tizio S. Corticosteroids and muscle wasting: role of transcription factors, nuclear cofactors, and hyperacetylation. Curr Opin Clin Nutr Metab Care. 2010;13:423-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). 2012;205:324-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 94. | Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 95. | Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 96. | Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. Int J Endocrinol. 2012;2012:127362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 98. | Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. J Appl Physiol. 2008;105:1772-1778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Ammary-Risch NJ, Huang SS. The primary care physician’s role in preventing vision loss and blindness in patients with diabetes. J Natl Med Assoc. 2011;103:281-283. [PubMed] [Cited in This Article: ] |
| 100. | Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34:33-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 101. | Payne JF, Ray R, Watson DG, Delille C, Rimler E, Cleveland J, Lynn MJ, Tangpricha V, Srivastava SK. Vitamin D insufficiency in diabetic retinopathy 2012. Endocr Pract. 2012;18:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33:2238-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |