Abstract
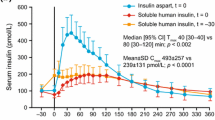
Standard or ‘traditional’ human insulin preparations such as regular soluble insulin and neutral protamine Hagedorn (NPH) insulin have shortcomings in terms of their pharmacokinetic and pharmacodynamic properties that limit their clinical efficacy. Structurally modified insulin molecules or insulin ‘analogs’ have been developed with the aim of delivering insulin replacement therapy in a more physiological manner. In the last 10 years, five insulin analog preparations have become commercially available for clinical use in patients with type 1 diabetes mellitus: three ‘rapid’ or fast-acting analogs (insulin lispro, aspart, and glulisine) and two long-acting analogs (insulin glargine and detemir). This review highlights the specific pharmacokinetic properties of these new insulin analog preparations and focuses on their potential clinical advantages and disadvantages when used in children and adolescents with type 1 diabetes mellitus.
The fast-acting analogs specifically facilitate more flexible insulin injection timing with regard to meals and activities, whereas the long-acting analogs have a more predictable profile of action and lack a peak effect. To date, clinical trials in children and adolescents have been few in number, but the evidence available from these and from other studies carried out in adults with type 1 diabetes suggest that they offer significant benefits in terms of reduced frequency of nocturnal hypoglycemia, better postprandial blood glucose control, and improved quality of life when compared with traditional insulins. In addition, insulin detemir therapy is unique in that patients may benefit from reduced risk of excessive weight, particularly during adolescence. Evidence for sustained long-term improvements in glycosylated hemoglobin, on the other hand, is modest. Furthermore, alterations to insulin/insulin-like growth factor I receptor binding characteristics have also raised theoretical concerns that insulin analogs may have an increased mitogenic potential and risk of tumor development, although evidence from both in vitro and in vivo animal studies do not support this assertion. Long-term surveillance has been recommended and further carefully designed prospective studies are needed to evaluate the overall benefits and clinical efficacy of insulin analog therapy in children and adolescents with type 1 diabetes.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Mosekilde E, Jensen KS, Binder C, et al. Modeling absorption kinetics of subcutaneous injected soluble insulin. J Pharmacokinet Biopharm 1989 Feb; 17(1): 67–87
Homko C, Deluzio A, Jimenez C, et al. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care 2003 Jul; 26(7): 2027–31
Becker RH, Frick AD, Burger F, et al. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005 Sep; 113(8): 435–43
Plank J, Wutte A, Brunner G, et al. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002 Nov; 25(11): 2053–7
Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40(9): 641–59
Howey DC, Bowsher RR, Brunelle RL, et al. [Lys(B28), Pro(B29)]-human insulin: a rapidly absorbed analogue of human insulin. Diabetes 1994 Mar; 43(3): 396–402
Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999 May; 55(3): 199–203
Danne T, Becker RH, Heise T, et al. Pharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetes. Diabetes Care 2005 Sep; 28(9): 2100–5
ter Braak EW, Woodworth JR, Bianchi R, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care 1996 Dec; 19(12): 1437–40
Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 Asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 1999 Sep; 22(9): 1501–6
Gardner DF, Arakaki RF, Podet EJ, et al. The pharmacokinetics of subcutaneous regular insulin in type I diabetic patients: assessment using a glucose clamp technique. J Clin Endocrinol Metab 1986; 63(3): 689–94
Mortensen HB, Lindholm A, Olsen BS, et al. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr 2000 Jul; 159(7): 483–8
Rutledge KS, Chase HP, Klingensmith GJ, et al. Effectiveness of postprandial Humalog in toddlers with diabetes. Pediatrics 1997 Dec; 100(6): 968–72
Kamoi K, Miyakoshi M, Maruyama R. A quality-of-life assessment of intensive insulin therapy using insulin lispro switched from short-acting insulin and measured by an ITR-QOL questionnaire: a prospective comparison of multiple daily insulin injections and continuous subcutaneous insulin infusion. Diabetes Res Clin Pract 2004 Apr; 64(1): 19–25
Grey M, Boland EA, Tamborlane WV. Use of lispro insulin and quality of life in adolescents on intensive therapy. Diabetes Educ 1999 Nov–Dec; 25(6): 934–41
Deja G, Jarosz-Chobot P, Muchacka-Bianga M, et al. Insulin lispro in treatment of children and adolescents with type 1 diabetes and its effect on quality of life. Diabetologia Polska 2002; 9(2): 52–6
Bott U, Ebrahim S, Hirschberger S, et al. Effect of the rapid-acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabet Med 2003 Aug; 20(8): 626–34
Anderson Jr JH, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Multicenter Insulin Lispro Study Group. Diabetes 1997 Feb; 46(2): 265–70
Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care 1998 Nov; 21(11): 1904–9
Gough SC. A review of human and analogue insulin trials. Diabetes Res Clin Pract 2007; 77(1): 1–15
American Diabetes Association. Postprandial blood glucose. Diabetes Care 2001 Apr; 24(4): 775–8
Ceriello A, Davidson J, Hanefeld M, et al. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis 2006 Oct; 16(7): 453–6
Amin R, Ross K, Acerini CL, et al. Hypoglycemia prevalence in prepubertal children with type 1 diabetes on standard insulin regimen: use of continuous glucose monitoring system. Diabetes Care 2003 Mar; 26(3): 662–7
Mohn A, Strang S, Wernicke-Panten K, et al. Nocturnal glucose control and free insulin levels in children with type 1 diabetes by use of the long-acting insulin HOE 901 as part of a three-injection regimen. Diabetes Care 2000 Apr; 23(4): 557–9
Ford-Adams ME, Murphy NP, Moore EJ, et al. Insulin lispro: a potential role in preventing nocturnal hypoglycaemia in young children with diabetes mellitus. Diabet Med 2003 Aug; 20(8): 656–60
Brunelle BL, Llewelyn J, Anderson Jr JH, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 1998 Oct; 21(10): 1726–31
Hermansen K, Fontaine P, Kukolja KK, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004 Apr; 47(4): 622–9
Home PD, Hallgren P, Usadel KH, et al. Pre-meal insulin aspart compared with pre-meal soluble human insulin in type 1 diabetes. Diabetes Res Clin Pract 2006 Feb; 71(2): 131–9
Raskin P, Guthrie RA, Leiter L, et al. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000 May; 23(5): 583–8
Plank J, Siebenhofer A, Berghold A, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005 Jun 27; 165(12): 1337–44
Garcia L, Lamas C, Tuset MJ, et al. Treatment with the insulin analogue lispro in children and adolescents with type 1 diabetes mellitus: evaluation over a 3-year period. Diabetes Nutr Metab 2002 Feb; 15(1): 7–13
Raskin P, Holcombe JH, Tamborlane WV, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications 2001 Nov–Dec; 15(6): 295–300
Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002 Mar; 25(3): 439–44
Tubiana-Rufi N, Coutant R, Bloch J, et al. Special management of insulin lispro in continuous subcutaneous insulin infusion in young diabetic children: a randomized cross-over study. Horm Res 2004; 62(6): 265–71
Della Manna T, Steinmetz L, Campos PR, et al. Subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care 2005 Aug; 28(8): 1856–61
Dunger DB, Sperling MA, Acerini CL, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics 2004 Feb; 113(2): e133–40
Mohn A, Matyka KA, Harris DA, et al. Lispro or regular insulin for multiple injection therapy in adolescence. Differences in free insulin and glucose levels overnight. Diabetes Care 1999 Jan; 22(1): 27–32
Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J 1996 Apr 1; 315 (Pt 1): 271–9
Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia 1997 Jul; 40Suppl. 2: S54–61
Bolli GB, Di Marchi RD, Park GD, Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 1999 Oct; 42(10): 1151–67
Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000 May; 23(5): 644–9
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000 Dec; 49(12): 2142–8
Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care 2000 Feb; 23(2): 157–62
Owens DR, Coates PA, Luzio SD, et al. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 2000 Jun; 23(6): 813–9
Heise T, Bott S, Rave K, et al. No evidence for accumulation of insulin glargine (LANTUS): a multiple injection study in patients with type 1 diabetes. Diabet Med 2002 Jun; 19(6): 490–5
Kurtzhals P, Havelund S, Jonassen I, et al. Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J 1995 Dec 15; 312 (Pt 3): 725–31
Whittingham JL, Havelund S, Jonassen I. Crystal structure of a prolonged-acting insulin with albumin-binding properties. Biochemistry 1997 Mar 11; 36(10): 2826–31
Home P, Kurtzhals P. Insulin detemir: from concept to clinical experience. Expert Opin Pharmacother 2006 Feb; 7(3): 325–43
Bott S, Tusek C, Jacobsen LV, et al. Insulin detemir under steady-state conditions: no accumulation and constant metabolic effect over time with twice daily administration in subjects with type 1 diabetes. Diabet Med 2006 May; 23(5): 522–8
Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res 2004 Aug; 21(8): 1498–504
Dea MK, Hamilton-Wessler M, Ader M, et al. Albumin binding of acylated insulin (NN304) does not deter action to stimulate glucose uptake. Diabetes 2002 Mar; 51(3): 762–9
Hordern SV, Wright JE, Umpleby AM, et al. Comparison of the effects on glucose and lipid metabolism of equipotent doses of insulin detemir and NPH insulin with a 16-h euglycaemic clamp. Diabetologia 2005 Mar; 48(3): 420–6
Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005 May; 28(5): 1107–12
Danne T, Lupke K, Walte K, et al. Insulin detemir is characterized by a consistent pharmacokinetic profile across age-groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care 2003 Nov; 26(11): 3087–92
Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004; 53: 1614–1620
Kurtzhals P, Havelund S, Jonassen I, et al. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J Pharm Sci 1997 Dec; 86(12): 1365–8
Matyka KA. Sweet dreams? Nocturnal hypoglycemia in children with type 1 diabetes. Pediatr Diabetes 2002 Jun; 3(2): 74–81
Murphy NP, Keane SM, Ong KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003 Mar; 26(3): 799–804
Tan CY, Wilson DM, Buckingham B. Initiation of insulin glargine in children and adolescents with type 1 diabetes. Pediatr Diabetes 2004 Jun; 5(2): 80–6
Chase HP, Dixon B, Pearson J, et al. Reduced hypoglycemic episodes and improved glycemic control in children with type 1 diabetes using insulin glargine and neutral protamine Hagedorn insulin. J Pediatr 2003 Dec; 143(6): 737–40
Colino E, Lopez-Capape M, Golmayo L, et al. Therapy with insulin glargine (Lantus) in toddlers, children and adolescents with type 1 diabetes. Diabetes Res Clin Pract 2005 Oct; 70(1): 1–7
Jackson A, Ternand C, Brunzell C, et al. Insulin glargine improves hemoglobin A1c in children and adolescents with poorly controlled type 1 diabetes. Pediatr Diabetes 2003 Jun; 4(2): 64–9
Dixon B, Chase HP, Burdick J, et al. Use of insulin glargine in children under age 6 with type 1 diabetes. Pediatr Diabetes 2005 Sep; 6(3): 150–4
Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr 1999 Apr; 134(4): 503–6
Robertson KJ, Schoenle E, Gucev Z, et al. Insulin detemir compared with NPH insulin in children and adolescents with type 1 diabetes. Diabet Med 2007 Jan; 24(1): 27–34
De Leeuw I, Vague P, Selam JL, et al. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab 2005 Jan; 7(1): 73–82
Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004 May; 27(5): 1081–7
Bryden KS, Neil A, Mayou RA, et al. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999 Dec; 22(12): 1956–60
Amiel SA, Caprio S, Sherwin RS, et al. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991 Feb; 72(2): 277–82
Standl E, Lang H, Roberts A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol Ther 2004 Oct; 6(5): 579–88
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes: causes, effects, and coping strategies. Diabetes Obes Metab 2007 Nov; 9(6): 799–812
Fiallo-Scharer R, Horner B, McFann K, et al. Mixing rapid-acting insulin analogues with insulin glargine in children with type 1 diabetes mellitus. J Pediatr 2006 Apr; 148(4): 481–4
Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000 Jun; 49(6): 999–1005
Ciaraldi TP, Carter L, Seipke G, et al. Effects of the long-acting insulin analog insulin glargine on cultured human skeletal muscle cells: comparisons to insulin and IGF-I. J Clin Endocrinol Metab 2001 Dec; 86(12): 5838–47
Stammberger I, Bube A, Durchfeld-Meyer B, et al. Evaluation of the carcinogenic potential of insulin glargine (LANTUS) in rats and mice. Int J Toxicol 2002 May–Jun; 21(3): 171–9
Slawik M, Schories M, Busse Grawitz A, et al. Treatment with insulin glargine does not suppress serum IGF-1. Diabet Med 2006 Jul; 23(7): 814–7
Acknowledgments
No sources of funding were used to assist in the preparation of this review. Carlo Acerini has received speaking/lecture honoraria and educational and research grant support for independent investigator-led research from Novo Nordisk. Harriet Miles has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miles, H.L., Acerini, C.L. Insulin Analog Preparations and Their Use in Children and Adolescents with Type 1 Diabetes Mellitus. Pediatr-Drugs 10, 163–176 (2008). https://doi.org/10.2165/00148581-200810030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00148581-200810030-00005