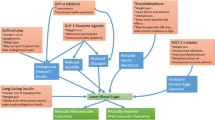
Abstract
Two landmark intervention studies, the Diabetes Control and Complications Trial (DCCT) in patients with type 1 diabetes mellitus and the United Kingdom Prospective Diabetes Study (UKPDS) in patients with type 2 diabetes mellitus, have unequivocally demonstrated that intensive diabetes therapy reduces the risk of long-term diabetic complications. As a result, the commonly accepted treatment goal for most patients with diabetes is the achievement and maintenance of glycemic control that is as close to the normal range as safely possible. Important adverse effects of intensive diabetes therapy, particularly when the treatment includes insulin or several of the oral antihyperglycemic agents, are an increased risk of hypoglycemia and undesired weight gain.
Improvement of glycemic control with insulin, insulin secretagogues (sulfonylureas, meglitinides), and insulin sensitizers (thiazolidinediones) is often accompanied by weight gain. The etiology of this weight gain is likely multifaceted, including a reduction of glucosuria, increased caloric intake to prevent hypoglycemia, and anabolic effects on adipose tissue.
Biguanides and α-glucosidase inhibitors have a neutral or even positive effect (decrease) on weight, which may partly be attributable to their non-insulinotropic mechanism of action, a modest effect on satiety, and to their gastrointestinal adverse effect profile.
Several antihyperglycemic agents that are currently in clinical development may improve glycemie control in conjunction with weight reduction. These include an analog of the pancreatic β-cell hormone amylin (pramlintide), as well as glucagon-like peptide-1 (GLP-1) and exendin, and their analogs.
Pharmacological agents with antihyperglycemic and positive weight effects have the potential to become important additions to our therapeutic armamentarium, in that they may help to achieve glycemie targets while addressing the long-standing clinical problem of weight gain as an adverse effect of intensive diabetes therapy.




Similar content being viewed by others
References
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14): 977–86
The Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996; 45(10): 1289–98
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131): 837–53
UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352(9131): 854–65
American Diabetes Association. Standards of medical care for patients with diabetes mellitus (position statement). Diabetes Care 2001 Jan; 24Suppl. 1: S33–43
ACE Consensus Conference on guidelines for glycemic control. American Association of Clinical Endocrinologists; 2001 Aug 20–21; Washington, DC
Felig P, Tamborlane W, Sherwin RS, et al. Insulin-infusion pump for diabetes. N Engl J Med 1979; 301: 1004–5
Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 1999; 42: 1151–67
DeFronzo RA. Pharmacologie therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131: 281–303
Hauner H. The impact of pharmacotherapy on weight management in type 2 diabetes. Int J Obes 1999; 23Suppl. 7: S12–7
The DCCT Research Group. Weight gain associated with intensive therapy in the diabetes control and complications trial: the DCCT Research Group. Diabetes Care 1988; 11(7): 567–73
The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care 2001; 24(10): 1711–21
Purnell JQ, Hokanson JE, Marcovina SM, et al. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT: Diabetes Control and Complications Trial. JAMA 1998; 280(2): 140–6
Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes 1994; 43: 313–7
Riddle MC. Should obese type 2 diabetic patients be treated with insulin? In: Gill GV, Pickup JC, Williams G, editors. Difficult diabetes. Oxford: Blackwell Science, 2001: 94–112
Henry RR, Gumbiner B, Ditzler T, et al. Intensive conventional insulin therapy for type II diabetes: metabolic effects during a 6-mo outpatient trial. Diabetes Care 1993; 16: 21–31
Makimattila S, Nikkila K, Yki-Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999 Apr; 42(4): 406–12
Yki-Jarvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001 Apr; 24(4): 758–67
Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999 Nov; 48(11): 2197–203
Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia 1999 Sep; 42(9): 1033–49
Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev 1986 Fall; 10(3): 295–315
Shimizu H, Tsuchiya T, Sato N, et al. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care 1998 Sep; 21(9): 1470–4
Gorson DM. Significant weight gain with rezulin therapy [letter]. Arch Intern Med 1999 Jan 11; 159(1): 99
Hirsch IB, Kelly J, Cooper S. Pulmonary edema associated with troglitazone therapy [letter]. Arch Intern Med 1999 Aug 9–23; 159(15): 1811
Saudek CD, Boulter PR, Knopp RH, et al. Sodium retention accompanying insulin treatment of diabetes mellitus. Diabetes 1974 Mar; 23(3): 240–6
Bryden KS, Neil A, Mayou RA, et al. Eating habits, body weight, and insulin misuse: a longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999 Dec; 22(12): 1956–60
American Diabetes Association. Intensive diabetes management. Alexandria (VA): American Diabetes Association, 1995: 48
Chow CC, Tsang LW, Sorensen JP, et al. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care 1995 Mar; 18(3): 307–14
Riddle MC, Schneider J. The Glimepiride Combination Group. Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Diabetes Care 1998; 21(7): 1052–7
Landstedt-Hallin L, Adamson U, Arner P, et al. Comparison of bedtime NPH or preprandial regular insulin combined with glibenclamide in secondary sulfonylurea failure. Diabetes Care 1995 Aug; 18(8): 1183–6
Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 2002 Apr; 25(4): 724–30
Gottlieb A, Velte M, Fineman M, et al. Pramlintide as an adjunct to insulin therapy improved glycemie and weight control in people with type 1 diabetes during treatment for 52 weeks [abstract]. Diabetes 2000; 49(1): A109
Campbell IW, Menzies DG, Chalmers J, et al. One year comparative trial of metformin and glipizide in type 2 diabetes mellitus. Diabet Metab 1994 Jul–Aug; 20(4): 394–400
Marbury T, Huang WC, Strange P, et al. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract 1999 Mar; 43(3): 155–66
Hermann LS, Schersten B, Bitzen PO, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations: a double-blind controlled study. Diabetes Care 1994 Oct; 17(10): 1100–9
Goldberg RB, Einhorn D, Lucas CP, et al. A randomized placebo-controlled trial of repaglinide in the treatment of type 2 diabetes. Diabetes Care 1998 Nov; 21(11): 1897–903
Horton ES, Clinkingbeard C, Gatlin M, et al. Nateglinide alone and in combination with metformin improves glycemie control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care 2000 Nov; 23(11): 1660–5
Starlix® (nateglinide tablets) package insert. Basel: Novartis International, 2000
Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemie control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000 Nov; 23(11): 1605–11
Lebovitz HE, Dole JF, Patwardhan R, et al. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 2001 Jan; 86(1): 280–8
Phillips LS, Grunberger G, Miller E, et al. The Rosiglitazone Clinical Trials Study Group. Once- and twice-daily dosing with rosiglitazone improves glycemie control in patients with type 2 diabetes. Diabetes Care 2001 Feb; 24(2): 308–15
DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulindependent diabetes mellitus. The MulticenterMetformin Study Group. N Engl J Med 1995; 333: 541–9
Glucophage® (metformin hydorchloride extended-release tablets), package insert. Princeton (NJ): Bristol-Myers Squibb Company, 2001
Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemie control over 3 years. UK Prospective Diabetes Study 44. Diabetes Care 1999 Jun; 22(6): 960–4
Segal P, Feig PU, Schernthaner G, et al. The efficacy and safety of miglitol therapy compared with glibenclamide in patients with NIDDM inadequately controlled by diet alone. Diabetes Care 1997 May; 20(5): 687–91
Johnston PS, Lebovitz HE, Coniff RF, et al. Advantages of alpha-glucosidase inhibition as monotherapy in elderly type 2 diabetic patients. J Clin Endocrinol Metab 1998 May; 83(5): 1515–22
Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemie control and complications in type II diabetes (VA CSDM): results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care 1995 Aug; 18(8): 1113–23
Yki-Jarvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992 Nov 12; 327(20): 1426–33
Clauson P, Karlander S, Steen L, et al. Daytime glibenclamide and bedtime NPH insulin compared to intensive insulin treatment in secondary sulphonylurea failure: a 1-year follow-up. Diabet Med 1996 May; 13(5): 471–7
Sonera IL, Agrawal L, Murphy JC, et al. Comparison of morning or bedtime insulin with and without glyburide in secondary sulfonylurea failure. Diabetes Care 1993 Jun; 16(6): 896–901
Yki-Jarvinen H, Ryysy L, Nikkila K, et al. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 1999 Mar 2; 130(5): 389–96
Howey DC, Bowsher RR, Brunelle RL, et al. [Lys (B28), Pro (B29)]-human insulin: a rapidly absorbed analogue of human insulin. Diabetes 1994 Mar; 43(3): 396–402
Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the longacting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000 May; 23(5): 644–9
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000 Dec; 49(12): 2142–8
Holleman F, Schmitt H, Rottiers R, et al. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. The Benelux-UK Insulin Lispro Study Group. Diabetes Care 1997 Dec; 20(12): 1827–32
Anderson Jr JH, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulinanalog treatment. Multicenter Insulin Lispro Study Group. Diabetes 1997 Feb; 46(2): 265–70
Anderson Jr JH, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Arch Intern Med 1997 Jun 9; 157(11): 1249–55
Vignati L, Anderson Jr JH, Iversen PW. Efficacy of insulin lispro in combination with NPH human insulin twice per day in patients with insulin-dependent or non-insulin-dependent diabetes mellitus: Multicenter Insulin Lispro Study Group. Clin Ther 1997 Nov–Dec; 19(6): 1408–21
Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care 2000 Feb; 23(2): 157–62
Rosenstock J, Park G, Zimmerman J, et al. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. US Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care 2000 Aug; 23(8): 1137–42
Ratner RE, Hirsch IB, Neifing JL, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care 2000 May; 23(5): 639–43
Yki-Jarvinen H, Dressier A, Ziemen M, et al. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 2000 Aug; 23(8): 1130–6
Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000 Nov; 23(11): 1666–71
Rosenstock J, Schwartz SL, Clark CM, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001 Apr; 24(4): 631–6
Schober E, Schoenle E, Van Dyk J, et al. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes. Diabetes Care 2001 Nov; 24(11): 2005–6
Pinget M, Jeandidier N. Long term safety and efficacy of intraperitoneal insulin infusion by means of implantable pumps. Horm Metab Res 1998 Aug; 30(8): 475–86
Saudek CD, Duckworth WC, Giobbie-Hurder A, et al. Implantable insulin pump vs multiple-dose insulin for non-insulin-dependent diabetes mellitus: a randomized clinical trial. Department of Veterans Affairs Implantable Insulin Pump Study Group. JAMA 1996 Oct 23–30; 276(16): 1322–7
Osei K, O’Dorisio TM, Falko JM. Concomitant insulin and sulfonylurea therapy in patients with type II diabetes: effects on glucoregulation and lipid metabolism. Am J Med 1984 Dec; 77(6): 1002–9
Lewitt MS, Yu VK, Rennie GC, et al. Effects of combined insulin-sulfonylurea therapy in type II patients. Diabetes Care 1989 Jun; 12(6): 379–83
Lins PE, Lundblad S, Persson-Trotzig E, et al. Glibenclamide improves the response to insulin treatment in non-insulin-dependent diabetics with second failure to sulfonylurea therapy. Acta Med Scand 1988; 223(2): 171–9
Schwartz S, Raskin P, Fonseca V, et al. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. N Engl J Med 1998 Mar 26; 338(13): 861–6
Actos® (pioglitazone hydrochloride tablets). Osaka: Takada Chemical Industries Ltd, 2002 (Data on file)
Raskin P, Rendell M, Riddle MC, et al. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care 2001 Jul; 24(7): 1226–32
Aviles-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 1999 Aug 3; 131(3): 182–8
Relimpio F, Pumar A, Losada F, et al. Adding metformin versus insulin dose increase in insulin-treated but poorly controlled type 2 diabetes mellitus: an open-label randomized trial. Diabet Med 1998 Dec; 15(12): 997–1002
Robinson AC, Burke J, Robinson S, et al. The effects of metformin on glycemic control and serum lipids in insulin-treated NIDDM patients with suboptimal metabolic control. Diabetes Care 1998 May; 21(5): 701–5
Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med 1994 Nov 14; 154(21): 2442–8
Ratner RE, Want LL, Fineman MS, et al. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther 2002; 4(1): 51–61
Fineman M, Gottlieb A, Skare S, et al. Pramlintide as an adjunct to insulin therapy improved glycemie and weight control in people with type 2 diabetes during treatment for 52 weeks [abstract]. Diabetes 2000; 49Suppl. 1: A106
Rosenstock J, Samols E, Muchmore DB, et al. Glimepiride, a new once-daily sulfonylurea: a double-blind placebo-controlled study of NIDDM patients. Glimepiride Study Group. Diabetes Care 1996; 19: 1194–9
Simonson DC, Kourides IA, Feinglos M, et al. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemie control and insulin secretion in NIDDM: results of two multicenter, randomized, placebo-controlled clinical trials. The Glipizide Gastrointestinal Therapeutic System Study Group. Diabetes Care 1997; 20: 597–606
Weyer C, Maggs DG, Young AA, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des 2001; 7(14): 1353–73
Young A. Amylin’s physiology and its role in diabetes. Curr Opin Endocrinol Diab 1997; 4: 282–90
Young A, Moore C, Herich J, et al. Neuroendocrine actions of amylin. In: Poyner D, Marshall I, Brain SD, editors. The CGRP family: calcitonin gene-related peptide (cgrp), amylin, and adrenomedullin. Georgetown (TX): Landes Bioscience, 2000: 91–102
Edelman SV, Weyer C. Unresolved challenges with insulin therapy in type 1 and type 2 diabetes: potential benefit of replacing amylin, a second β-cell hormone. Diabetes Technol Ther 2002; 4(2): 175–89
Lutz TA, Mollet A, Rushing PA, et al. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord 2001; 25(7): 1005–11
Rushing PA, Hagan MM, Seeley RJ, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 2001; 142(11): 5035–8
Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001 Aug; 44(8): 929–45
Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999 Sep; 104(6): 787–94
Luzi L, DeFronzo RA. Effect of loss of first-phase of insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am J Physiol 1989; 257: E241–6
Bruce DG, Chrisholm DJ, Storlien LH, et al. Physiological importance of deficiency in early prandial insulin secretion in non-insulin-dependent diabetes. Diabetes 1988; 37: 736–44
Dornhorst A. Insulinotropic meglitinide analogues. Lancet 2001 Nov 17; 358(9294): 1709–16
Owens DR. Repaglinide: a new short-acting insulinotropic agent for the treatment of type 2 diabetes. Eur J Clin Invest 1999 Jun; 29Suppl. 2: 30–7
Prandin® (repaglinide tablets) package insert. Princeton (NJ): Novo Nordisk Pharmaceuticals, Inc, 2002
Landgraf R, Frank M, Bauer C, et al. Prandial glucose regulation with repaglinide: its clinical and lifestyle impact in a large cohort of patients with type 2 diabetes. Int J Obes Relat Metab Disord 2000 Sep; 24Suppl. 3: S38–44
Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet 1999 Jul 10; 354(9173): 141–8
Saltiel AR, Olefsky JM. Thiazolindinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996; 45: 1661–9
Spiegelman BM. PPAR-γ: adipogenic regulator and thiazolizinedione receptor. Diabetes 1998; 47: 507–14
Actos® (pioglitazone hydrochloride tablets) package insert. Osaka: Takada Chemical Industries Ltd, 2002
Avandia® (rosiglitazone maleate) package insert. Philadelphia (PA): SmithKline Beecham Pharmaceuticals, 2001
Kumar S, Boulton AJ, Beck-Nielsen H, et al. Troglitazone, an insulin action enhancer, improves metabolic control in NIDDM patients. Troglitazone Study Group. Diabetologia 1996 Jun; 39(6): 701–9
Iwamoto Y, Kosaka K, Kuzuya T, et al. Effects of troglitazone: a new hypoglycemic agent in patients with NIDDM poorly controlled by diet therapy. Diabetes Care 1996; 19(2): 151–6
Fonseca V, Rosenstock J, Patwardhan R, et al. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA 2000 Apr 5; 283(13): 1695–702
Amylin Pharmaceuticals Inc. (Data on file)
Kolterman OG, Insel J, Saekow M, et al. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 1980 Jun; 65(6): 1272–84
Hollenbeck CB, Chen YD, Reaven GM. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes 1984 Jul; 33(7): 622–6
Olefsky J, Reaven GM, Farquhar JW. Effects of weight reduction on obesity: studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest 1974 Jan; 53(1): 64–76
Colman E, Katzel LI, Rogus E, et al. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism 1995 Nov; 44(11): 1502–8
Niskanen L, Uusitupa M, Sarlund H, et al. The effects of weight loss on insulin sensitivity, skeletal muscle composition and capillary density in obese nondiabetic subjects. Int J Obes Relat Metab Disord 1996 Feb; 20(2): 154–60
Danforth Jr E. Failure of adipocyte differentiation causes type II diabetes mellitus? [letter]. Nat Genet 2000 Sep; 26(1): 13
Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 2001 Nov 2; 276(44): 41245–54
Kawai T, Takei I, Oguma Y, et al. Effects of troglitazone on fat distribution in the treatment of male type 2 diabetes. Metabolism 1999 Sep; 48(9): 1102–7
Kelly IE, Han TS, Walsh K, et al. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 1999 Feb; 22(2): 288–93
Akazawa S, Sun F, Ito M, et al. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 2000 Aug; 23(8): 1067–71
Sparrow D, Borkan GA, Gerzof SG, et al. Relationship of fat distribution to glucose tolerance: results of computed tomography in male participants of the Normative Aging Study. Diabetes 1986 Apr; 35(4): 411–5
Fujioka S, Matsuzawa Y, Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987 Jan; 36(1): 54–9
Despres JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 1989 Mar; 38(3): 304–9
Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res 1998 Jan; 6(1): 47–53
Vague P, Juhan-Vage I, Alessi MC, et al. The effect of metformin on the metabolic anomalies associated with android type body fat distribution: results of the BIGPRO trial [abstract]. Diabetologia 1994; 37Suppl. 1: 236.
Lebovitz HE. α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev 1998; 6(2): 132–45
Precose® (acarbose tablets) package insert. West Haven (CT): Bayer Corporation Pharmaceuticals, 2001
Chiasson JL, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus: a multicenter controlled clinical trial. Ann Intern Med 1994 Dec 15; 121(12): 928–35
Hotta N, Kakuta H, Sano T, et al. Long-term effect of acarbose on glycaemic control in non-insulin-dependent diabetes mellitus: a placebo-controlled double-blind study. Diabet Med 1993 Mar; 10(2): 134–8
Hanefeld M, Fischer S, Schulze J, et al. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with diet alone. Diabetes Care 1991 Aug; 14(8): 732–7
Wolever TM, Chiasson JL, Josse RG, et al. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes Relat Metab Disord 1997 Sep; 21(9): 756–63
Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study. Am J Med 1997 Dec; 103(6): 483–90
Coniff RF, Shapiro JA, Seaton TB, et al. Multicenter, placebo-controlled trial comparing acarbose (BAY g 5421) with placebo, tolbutamide, and tolbutamideplus-acarbose in non-insulin-dependent diabetes mellitus. Am J Med 1995 May; 98(5): 443–51
Toeller M. Modulation of intestinal glucose absorption: postponement of glucose absorption by alpha-glucosidase inhibitors. In: Mogensen CE, Standl I, editors. Pharmacology of diabetes. Berlin: de Gruyter, 1991: 93–112
Lindstrom J, Tuomilehto J, Spengler M. Acarbose treatment does not change the habitual diet of patients with type 2 diabetes mellitus. The Finnish Acarbos Study Group. Diabet Med 2000 Jan; 17(1): 20–5
Fukase N, Takahashi H, Manaka H, et al. Differences in glucagon-like peptide-1 and GIP responses following sucrose ingestion. Diabetes Res Clin Pract 1992 Mar; 15(3): 187–95
Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 1988 Mar; 35(3): 214–43
Glick Z, Bray GA. Effects of acarbose on food intake, body weight and fat depots in lean and obease rats. Pharmacol Biochem Behav 1983 Jul; 19(1): 71–8
William-Olsson T. alpha-Glucosidase inhibition in obesity. Acta Med Scand Suppl 1985; 706: 1–39
Cooper GJ, Willis AC, Clark A, et al. Purification and characterization of apeptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A 1987; 84(23): 8628–32
Fineman MS, Giotta MP, Thompson RG, et al. Amylin response following Sustacal® ingestion is diminished in type II diabetic patients treated with insulin [abstract 566]. Diabetologia 1996; 39Suppl. 1: A149
Koda JE, Fineman M, Rink TJ, et al. Amylin concentrations and glucose control. Lancet 1992; 339(8802): 1179–80
Kolterman OG, Schwartz S, Corder C, et al. Effect of 14 days’ subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia 1996; 39(4): 492–9
Thompson RG, Peterson J, Gottlieb A, et al. Effects of pramlintide, an analog of human amylin, on plasma glucose profiles in patients with IDDM: results of a multicenter trial. Diabetes 1997; 46(4): 632–6
Thompson RG, Gottlieb A, Organ K, et al. Pramlintide: a human amylin analogue reduced postprandial plasma glucose, insulin and c-peptide concentrations in patients with type II diabetes. Diabet Med 1997; 14(7): 547–55
Kolterman OG, Gottlieb A, Moyses C, et al. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC137, a human amylin analogue. Diabetes Care 1995; 18(8): 1179–82
Thompson RG, Pearson L, Schoenfeld SL, et al. Pramlintide, a synthetic analog of human amylin, improves the metabolic profile of patients with type 2 diabetes using insulin. The Pramlintide in Type 2 Diabetes Group. Diabetes Care 1998; 21(6): 987–93
Fineman MS, Koda JE, Shen LZ, et al. The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism. 2002 May; 51(5): 636–41
Fineman M, Organ K, Kolterman O. The human amylin analogue pramlintide suppressed glucagon secretion in patients with type 2 diabetes [abstract]. Diabetologia 1998; 41: A167
Nyholm B, Orskov L, Hove K, et al. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism 1999; 48(7): 935–41
Kong MF, King P, Macdonald IA, et al. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia 1997; 40: 82–8
Kong MF, Stubbs TA, King P, et al. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia 1998; 41(5): 577–83
Rushing PA, Hagan MM, Seeley RJ, et al. Amylin: a novel action in the brain to reduce body weight. Endocrinology 2000; 141(2): 850–3
Rushing PA, Lutz TA, Seeley RJ, et al. Amylin and insulin interact to reduce food intake in rats. Horm Metab Res 2000; 32: 62–5
Fineman M, Maggs D, Burcell T, et al. Addition of pramlintide to insulin therapy in type 1 diabetes: impact on glycemic and weight control stratified by BMI [abstract]. Diabetes 2001; 50Suppl. 2: A112
Weyer C, Maggs DG, Fineman MS, et al. the human amylin analog, pramlintide, reduces body weight in insulin-treated patients with type 2 diabetes. 9th Inter-National Congress on Obesity; 2002 Aug 24–29; Sao Paulo, Brazil.
Drucker DJ. Development of glucagon-like peptide-1-based Pharmaceuticals as therapeutic agents for the treatment of diabetes. Curr Pharm Des 2001 Sep; 7(14): 1399–412
Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology 2001; 142(2): 521–7
Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic â-cell function. Eur J Endocrinol 2000; 143: 717–25
Baron AD, Kim D, Weyer C. Novel peptides under development for the treatment of type 1 and type 2 diabetes mellitus. Curr Drug Targets Immune Endocr Metab Disord 2002; 2(1): 63–82
Rachman J, Barrow BA, Levy JC, et al. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia 1997; 40: 205–11
Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996 Jan 4; 379(6560): 69–72
Flint A, Raben A, Ersboll AK, et al. The effect of physiological levels of glucagonlike peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001 Jun; 25(6): 781–92
Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 1999 Jul; 22(7): 1137–43
Bhavsar SP, Watkins JJ, Young AA. Central and peripheral administration of exendin-4 reduces food intake in rats [abstract 828]. Diabetologia 1998; 41Suppl. 1: A214
Kieffer TJ, Huang Z, Mclntosh CH, et al. Gastric inhibitory polypeptide release from a tumor-derived cell line. Am J Physiol 1995 Aug; 269 (2 Pt 1): E316–22
Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes 1998 Nov; 47(11): 1663–70
Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a longacting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 2002 Feb; 51(2): 424–9
Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001 Jul; 281(1): E155–61
Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and â-cell function in type 2 diabetes: a parallel-group study. Lancet 2002 Mar 9; 359: 824–30
Fineman M, Bicsak T, Shen L, et al. AC2993 (synthetic exendin-4) improved glycemie control in patients with type 2 diabetes during 28 days of treatment in a multicenter, randomized, triple-blind, placebo-controlled study. American Diabetes Association, 61st Scientific Sessions; 2001 Jun 22–26; Philadelphia (PA). 36
Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagonlike peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 2001 Sep; 86(9): 4382–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purnell, J.Q., Weyer, C. Weight Effect of Current and Experimental Drugs for Diabetes Mellitus. Mol Diag Ther 2, 33–47 (2003). https://doi.org/10.2165/00024677-200302010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200302010-00004