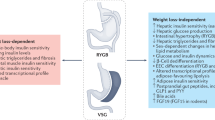
Abstract
Several conventional methods of bariatric surgery can induce long-term remission of type 2 diabetes mellitus (T2DM); novel gastrointestinal surgical procedures are reported to have similar effects. These procedures also dramatically improve other metabolic conditions, including hyperlipidemia and hypertension, in both obese and nonobese patients. Several studies have provided evidence that these metabolic effects are not simply the results of drastic weight loss and decreased caloric intake but might be attributable, in part, to endocrine changes resulting from surgical manipulation of the gastrointestinal tract. In this Review, we provide an overview of the clinical evidence that demonstrates the effects of such interventions—termed metabolic surgery—on T2DM and discuss the implications for future research. In light of the evidence presented here, we speculate that the gastrointestinal tract might have a role in the pathophysiology of T2DM and obesity.
Key Points
-
Conventional and novel bariatric surgeries induce long-term remission of type 2 diabetes mellitus (T2DM) and dramatically improve other metabolic conditions, including hyperlipidemia and hypertension
-
Animal studies and clinical investigations show that the effects of surgery on T2DM might be partly explained by endocrine changes that result from surgical manipulation of the gastrointestinal tract
-
Current BMI-based criteria for patient selection are not sufficiently inclusive to define indications for surgical treatment and evaluation of the risk profile of patients with T2DM
-
Randomized, controlled trials that compare surgery with medical treatment should aim to define the role of surgery in the management of T2DM and identify new criteria for selection
-
Research into the mechanisms of action of metabolic surgery represents an extraordinary opportunity to improve our understanding of the pathophysiology of T2DM and ultimately improve its treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Buchwald, H. et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 292, 1724–1737 (2004).
Cohen, R., Pinheiro, J. S., Correa, J. L. & Schiavon, C. A. Laparoscopic Roux-en-Y gastric bypass for BMI <35 kg/m2: a tailored approach. Surg. Obes. Relat. Dis. 2, 401–404 (2006).
Lee, W. J. et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI >35 and <35 kg/m2. J. Gastrointest. Surg. 12, 945–952 (2008).
O'Brien, P. E. et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann. Intern. Med. 144, 625–633 (2006).
Suter, M., Calmes, J. M., Paroz, A. Romy, A. & Giusti, V. Results of Roux-en-Y gastric bypass in morbidly obese vs super obese patients: similar body weight loss, correction of comorbidities, and improvement of quality of life. Arch. Surg. 144, 312–318 (2009).
Thaler, J. P. & Cummings, D. E. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150, 2518–2525 (2009).
Sjöström, L. et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Dixon, J. B. et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299, 316–323 (2008).
Pories, W. J. et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann. Surg. 222, 339–350 (1995).
Schauer, P. R. et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 238, 467–484 (2003).
Scopinaro, N. Biliopancreatic diversion: mechanisms of action and long-term results. Obes. Surg. 16, 683–689 (2006).
Vidal, J. et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes. Surg. 18, 1077–1082 (2008).
Gan, S. S., Talbot, M. L. & Jorgensen, J. O. Efficacy of surgery in the management of obesity-related type 2 diabetes mellitus. ANZ J. Surg. 77, 958–962 (2007).
Chiellini, C., Rubino, F., Castagneto, M., Nanni, G. & Mingrone, G. The effect of bilio-pancreatic diversion on type 2 diabetes in patients with BMI <35 kg/m2. Diabetologia 52, 1027–1030 (2009).
Noya, G. et al. Biliopancreatic diversion preserving the stomach and pylorus in the treatment of hypercholesterolemia and diabetes type II: results in the first 10 cases. Obes. Surg. 8, 67–72 (1998).
Scopinaro, N., Marinari, G. M., Camerini, G. B., Papadia, F. S. & Adami, G. F. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care 28, 2406–2411 (2005).
Cohen, R. V., Schiavon, C. A., Pinheiro, J. S., Correa, J. L. & Rubino, F. Duodenal–jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg. Obes. Relat. Dis. 3, 195–197 (2007).
Ramos, A. C. et al. Laparoscopic duodenal–jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI <30 kg/m² (LBMI). Obes. Surg. 19, 307–312 (2009).
Ferzli, G. S. et al. Clinical improvement after duodenojejunal bypass for non obese type 2 diabetes despite minimal improvement in glycemic homeostasis. World J. Surg. 33, 972–979 (2009).
DePaula, A. L. et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg. Endosc. 22, 706–716 (2008).
DePaula, A. L., Macedo, A. L., Mota, B. R. & Schraibman, V. Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg. Endosc. 23, 1313–1320 (2009).
Aguirre, V., Stylopoulos, N., Grinbaum, R. & Kaplan, L. M. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity 16, 2585–2592 (2008).
Rodriguez-Grunert, L. et al. First human experience with endoscopically delivered and retrieved duodenal–jejunal bypass sleeve. Surg. Obes. Relat. Dis. 4, 55–59 (2008).
MacDonald, K. G. Jr et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J. Gastrointest. Surg. 1, 213–220 (1997).
Flum, D. R. & Dellinger, E. P. Impact of gastric bypass operation on survival: a population-based analysis. J. Am. Coll. Surg. 199, 543–551 (2004).
Christou, N. V. et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann. Surg. 240, 416–423 (2004).
Sowemimo, O. A. et al. Natural history of morbid obesity without surgical intervention. Surg. Obes. Relat. Dis. 3, 73–77 (2007).
Peeters, A. et al. Substantial intentional weight loss and mortality in the severely obese. Ann. Surg. 246, 1028–1033 (2007).
Perry, C. D., Hutter, M. M., Smith, D. B., Newhouse, J. P. & McNeil, B. J. Survival and changes in comorbidities after bariatric surgery. Ann. Surg. 247, 21–27 (2008).
Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357, 753–761 (2007).
Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Buchwald, H., Estok, R., Fahrbach, K., Bane, D. & Sledge, I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 142, 621–632 (2007).
Wittgrove, A. C. & Clark, G. W. Laparoscopic gastric bypass, Roux-en-Y 500 patients: technique and results, with 3–60 month follow-up. Obes. Surg. 10, 233–239 (2000).
Morínigo, R. et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes. Surg. 16, 1594–1601 (2006).
Wickremesekera, K., Miller, G., Naotunne, T. D., Knowles, G. & Stubbs, R. S. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes. Surg. 15, 474–481 (2005).
Pontiroli, A. E. et al. Laparoscopic gastric banding prevents type 2 diabetes and arterial hypertension and induces their remission in morbid obesity: a 4-year case-controlled study. Diabetes Care 28, 2703–2709 (2005).
LABS Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 361, 445–454 (2009).
Khuri, S. F. et al. Comparison of surgical outcomes between teaching and non-teaching hospitals in the Department of Veterans Affairs. Ann. Surg. 234, 370–382 (2001).
Encinosa, W. E., Bernard, D. M., Du, D. & Steiner, C. A. Recent improvements in bariatric surgery outcomes. Med. Care 47, 531–535 (2009).
Nguyen, N. T., Hinojosa, M., Fayad, C., Varela, E. & Wilson, S. E. Use and outcomes of laparoscopic versus open gastric bypass at academic and medical centers. J. Am. Coll. Surg. 205, 248–255 (2007).
Han, S. H. et al. Improved outcomes using a systematic and evidence based approach to the laparoscopic Roux-en-Y gastric bypass in a single academic institution. Am. Surg. 73, 955–958 (2007).
Mari, A. et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia 49, 2136–2143 (2006).
Patti, M. E. et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48, 2236–2240 (2005).
Polyzogopoulou, E. V., Kalfarentzos, F., Vagenakis, A. G. & Alexandrides, T. K. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52, 1098–1103 (2003).
Laferrère, B. et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 93, 2479–2485 (2008).
Rubino, F. & Marescaux, J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann. Surg. 239, 1–11 (2004).
Kindel, T. L., Yoder, S. M., Seeley, R. J., D'Alessio, D. A. & Tso, P. Duodenal–jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J. Gastrointest. Surg. 13, 1762–1772 (2009).
Pacheco, D. et al. The effects of duodenal–jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am. J. Surg. 194, 221–224 (2007).
Wang, T. T. et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann. Surg. 247, 968–975 (2008).
Troy, S. et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell. Metab. 8, 201–211 (2008).
Strader, A. D., Clausen, T. R., Goodin, S. Z. & Wendt, D. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes. Surg. 19, 96–104 (2009).
Pattou, F. et al. Restoration of beta cell function after bariatric surgery in type 2 diabetes patients: A prospective controlled study comparing gastric banding and gastric bypass. Obes. Surg. 17, 1041–1043 (2007).
Pattou, F. et al. Catering of insulin secretion after a gastric bypass in type 2 diabetes is independent from weight loss and correlated to the increase of GLP. Diabetes Metab. 34, A23 (2008).
Cummings, D. E., Overduin, J., Foster-Schubert, K. E. & Carlson, M. J. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg. Obes. Relat. Dis. 3, 109–115 (2007).
Moo, T. A. & Rubino, F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 15, 153–158 (2008).
Damholt, A. B., Buchan, A. M. & Kofod, H. Glucagon-like-peptide-1 secretion from canine L-cells is increased by glucose-dependent-insulinotropic peptide but unaffected by glucose. Endocrinology 139, 2085–2091 (1998).
Rubino, F. et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann. Surg. 2, 236–242 (2004).
le Roux, C. W. et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 243, 108–114 (2006).
le Roux, C. W. et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 246, 780–785 (2007).
Korner, J. et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity 14, 1553–1561 (2006).
Korner, J. et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab. 90, 359–365 (2005).
Cummings, D. E. et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 346, 1623–1630 (2002).
Laferrère, B. et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30, 1709–1716 (2007).
NIH Conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann. Intern. Med. 115, 956–961 (1991).
Pasarica, M. & Dhurandhar, N. V. Infectobesity: obesity of infectious origin. Adv. Food Nutr. Res. 52, 61–102 (2007).
Burcelin, R., Luche, E., Serino, M. & Amar, J. The gut microbiota ecology: a new opportunity for the treatment of metabolic diseases? Front. Biosci. 14, 5107–5117 (2009).
Lovshin, J. A. & Drucker, D. J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5, 262–269 (2009).
Rubino, F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 31 (Suppl. 2), S290–S296 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Francesco Rubino declares associations with the following companies: Covidien (speakers bureau), Ethicon Endo-Surgery (speakers bureau), GI Dynamics (consultant), NGM Biopharmaceuticals (consultant) and Roche (research support).
Timothy E. McGraw declares associations with the following companies: Hoffman-La Roche (research support) and Sanofi Aventis (research support).
Sarah L. R'Bibo, Federica del Genio and Madhu Mazumdar declare no competing interests.
Rights and permissions
About this article
Cite this article
Rubino, F., R'bibo, S., del Genio, F. et al. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol 6, 102–109 (2010). https://doi.org/10.1038/nrendo.2009.268
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.268
This article is cited by
-
Dietary excess regulates absorption and surface of gut epithelium through intestinal PPARα
Nature Communications (2021)
-
The Importance of Intestinal Length in Triglyceride Metabolism and in Predicting the Outcomes of Comorbidities in Laparoscopic Roux-en-Y Gastric Bypass—a Narrative Review
Obesity Surgery (2021)
-
Duodenal-Jejunal Bypass Ameliorates Type 2 Diabetes Mellitus by Activating Insulin Signaling and Improving Glucose Utilization in the Brain
Obesity Surgery (2020)
-
The Effect of Gastric Bypass with a Distal Gastric Pouch on Glucose Tolerance and Diabetes Remission in Type 2 Diabetes Sprague-Dawley Rat Model
Obesity Surgery (2019)
-
The Impact of Laparoscopic Sleeve Gastrectomy with Duodenojejunal Bypass on Intestinal Microbiota Differs from that of Laparoscopic Sleeve Gastrectomy in Japanese Patients with Obesity
Clinical Drug Investigation (2018)