Abstract
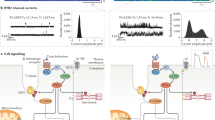
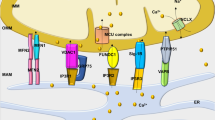
Cardiac myocyte function is dependent on the synchronized movements of Ca2+ into and out of the cell, as well as between the cytosol and sarcoplasmic reticulum. These movements determine cardiac rhythm and regulate excitation–contraction coupling. Ca2+ cycling is mediated by a number of critical Ca2+-handling proteins and transporters, such as L-type Ca2+ channels (LTCCs) and sodium/calcium exchangers in the sarcolemma, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2a), ryanodine receptors, and cardiac phospholamban in the sarcoplasmic reticulum. The entry of Ca2+ into the cytosol through LTCCs activates the release of Ca2+ from the sarcoplasmic reticulum through ryanodine receptor channels and initiates myocyte contraction, whereas SERCA2a and cardiac phospholamban have a key role in sarcoplasmic reticulum Ca2+ sequesteration and myocyte relaxation. Excitation–contraction coupling is regulated by phosphorylation of Ca2+-handling proteins. Abnormalities in sarcoplasmic reticulum Ca2+ cycling are hallmarks of heart failure and contribute to the pathophysiology and progression of this disease. Correcting impaired intracellular Ca2+ cycling is a promising new approach for the treatment of heart failure. Novel therapeutic strategies that enhance myocyte Ca2+ homeostasis could prevent and reverse adverse cardiac remodeling and improve clinical outcomes in patients with heart failure.
Key Points
-
Ca2+ cycling defects in cardiac myocytes are a hallmark of heart failure
-
Ca2+-handling abnormalities in failing cardiac myocytes include reduced Ca2+ uptake, decreased sarcoplasmic reticulum Ca2+ sequestration, and defective Ca2+ release from the sarcoplasmic reticulum, resulting in cytosolic Ca2+ overload
-
Changes in the expression and activity of Ca2+-handling proteins have been described in patients with chronic heart failure
-
Restoration of sarcoplasmic reticulum Ca2+ uptake through activation of sarcoplasmic/endoplasmic reticulum calcium ATPase 2a is a promising strategy for the treatment of patients with heart failure
-
Reducing Ca2+ leakage from the sarcoplasmic reticulum by targeting calcium/calmodulin-dependent protein kinase type II or stabilizing ryanodine receptors is also a promising strategy for treatment of patients with heart failure
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
de Giuli, F. et al. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur. J. Heart Fail. 7, 295–302 (2005).
Sliwa, K., Damasceno, A. & Mayosi, B. M. Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112, 3577–3583 (2005).
Jiang, H. & Ge, J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart 95, 1727–1731 (2009).
Clapham, D. E. Calcium signaling. Cell 80, 259–268 (1995).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. Seven transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002).
Antos, C. L. et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ. Res. 89, 997–1004 (2001).
Feldman, R. D. & Gros, R. New insights into the regulation of cAMP synthesis beyond GPCR/G protein activation: implications in cardiovascular regulation. Life Sci. 81, 267–271 (2007).
Wittkopper, K., Dobrev, D., Eschenhagen, T. & El-Armouche, A. Phosphatase-1 inhibitor-1 in physiological and pathological β-adrenoceptor signalling. Cardiovasc. Res. 91, 392–401 (2011).
Reinkober, J. et al. Targeting GRK2 by gene therapy for heart failure: benefits above β-blockade. Gene Ther. 19, 686–693 (2012).
Sculptoreanu, A., Rotman, E., Takahashi, M., Scheuer, T. & Catterall, W. A. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 90, 10135–10139 (1993).
Kamp, T. J. & Hell, J. W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102 (2000).
Colyer, J. & Wang, J. H. Dependence of cardiac sarcoplasmic reticulum calcium pump activity on the phosphorylation status of phospholamban. J. Biol. Chem. 266, 17486–17493 (1991).
Haghighi, K., Gregory, K. N. & Kranias, E. G. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 322, 1214–1222 (2004).
Chen, Z., Akin, B. L. & Jones, L. R. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J. Biol. Chem. 282, 20968–20976 (2007).
Li, L., Desantiago, J., Chu, G., Kranias, E. G. & Bers, D. M. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. Am. J. Physiol. Heart Circ. Physiol. 278, H769–H779 (2000).
Kentish, J. C. et al. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ. Res. 88, 1059–1065 (2001).
Pena, J. R. & Wolska, B. M. Troponin I phosphorylation plays an important role in the relaxant effect of β-adrenergic stimulation in mouse hearts. Cardiovasc. Res. 61, 756–763 (2004).
Lohse, M. J., Engelhardt, S. & Eschenhagen, T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 (2003).
Lyon, A. R. et al. Plasticity of surface structures and β2-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ. Heart Fail. 5, 357–365 (2012).
Tilley, D. G. & Rockman, H. A. Role of β-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev. Cardiovasc. Ther. 4, 417–432 (2006).
Ho, D., Yan, L., Iwatsubo, K., Vatner, D. E. & Vatner, S. F. Modulation of β-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail. Rev. 15, 495–512 (2010).
Perreault, C. L., Bing, O. H., Brooks, W. W., Ransil, B. J. & Morgan, J. P. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ. Res. 67, 707–712 (1990).
Ungerer, M., Bohm, M., Elce, J. S., Erdmann, E. & Lohse, M. J. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation 87, 454–463 (1993).
Kiuchi, K. et al. Myocardial β-adrenergic receptor function during the development of pacing-induced heart failure. J. Clin. Invest. 91, 907–914 (1993).
Choi, D. J., Koch, W. J., Hunter, J. J. & Rockman, H. A. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J. Biol. Chem. 272, 17223–17229 (1997).
Woo, A. Y. & Xiao, R. P. β-adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol. Sin. 33, 335–341 (2012).
Grimm, M. & Brown, J. H. β-adrenergic receptor signaling in the heart: role of CaMKII. J. Mol. Cell Cardiol. 48, 322–330 (2009).
Wilkins, B. J. et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94, 110–118 (2004).
Zhang, C. L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Voelkers, M. et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J. Mol. Cell Cardiol. 48, 1329–1334 (2010).
Hulot, J. S. et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 124, 796–805 (2011).
Buraei, Z. & Yang, J. The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 (2010).
Andrade, A. et al. The α2δ subunit augments functional expression and modifies the pharmacology of CaV1.3 L-type channels. Cell Calcium 46, 282–292 (2009).
Benitah, J. P., Alvarez, J. L. & Gomez, A. M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell Cardiol. 48, 26–36 (2010).
Seisenberger, C. et al. Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 275, 39193–39199 (2000).
Weissgerber, P. et al. Reduced cardiac L-type Ca2+ current in CaVβ2-/- embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ. Res. 99, 749–757 (2006).
Semsarian, C. et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J. Clin. Invest. 109, 1013–1020 (2002).
Liao, Y. et al. Benidipine, a long-acting calcium channel blocker, inhibits cardiac remodeling in pressure-overloaded mice. Cardiovasc. Res. 65, 879–888 (2005).
Mukherjee, R. & Spinale, F. G. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J. Mol. Cell Cardiol. 30, 1899–1916 (1998).
Goonasekera, S. A. et al. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J. Clin. Invest. 122, 280–290 (2012).
Schroder, F. et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 98, 969–976 (1998).
Chen, X. et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 91, 517–524 (2002).
Mahe, I., Chassany, O., Grenard, A. S., Caulin, C. & Bergmann, J. F. Defining the role of calcium channel antagonists in heart failure due to systolic dysfunction. Am. J. Cardiovasc. Drugs 3, 33–41 (2003).
Luo, X. et al. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 52, 136–147 (2012).
Parekh, A. B. & Putney, J. W. Jr. Store-operated calcium channels. Physiol. Rev. 85, 757–810 (2005).
Vig, M. et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006).
Prakriya, M. et al. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 (2006).
Liou, J. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005).
Roos, J. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005).
Zhang, S. L. et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl Acad. Sci. USA 103, 9357–9362 (2006).
Stathopulos, P. B., Zheng, L., Li, G. Y., Plevin, M. J. & Ikura, M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135, 110–122 (2008).
Luik, R. M., Wu, M. M., Buchanan, J. & Lewis, R. S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 (2006).
Stathopulos, P. B., Li, G. Y., Plevin, M. J., Ames, J. B. & Ikura, M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 281, 35855–35862 (2006).
Wu, M. M., Buchanan, J., Luik, R. M. & Lewis, R. S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 (2006).
Park, C. Y. et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009).
Gwack, Y. et al. A genome-wide drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441, 646–650 (2006).
Aubart, F. C. et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol. Ther. 17, 455–462 (2009).
Kurebayashi, N. & Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 533, 185–199 (2001).
Pan, Z. et al. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 4, 379–383 (2002).
Launikonis, B. S., Barnes, M. & Stephenson, D. G. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl Acad. Sci. USA 100, 2941–2944 (2003).
Gibson, A., McFadzean, I., Wallace, P. & Wayman, C. P. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol. Sci. 19, 266–269 (1998).
Leung, F. P., Yung, L. M., Yao, X., Laher, I. & Huang, Y. Store-operated calcium entry in vascular smooth muscle. Br. J. Pharmacol. 153, 846–857 (2008).
Zanou, N. et al. Role of TRPC1 channel in skeletal muscle function. Am. J. Physiol. Cell Physiol. 298, C149–C162 (2010).
Ohba, T. et al. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem. Biophys. Res. Commun. 389, 172–176 (2009).
Mulieri, L. A., Hasenfuss, G., Leavitt, B., Allen, P. D. & Alpert, N. R. Altered myocardial force-frequency relation in human heart failure. Circulation 85, 1743–1750 (1992).
Pieske, B. et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation 92, 1169–1178 (1995).
Mercadier, J. J. et al. Altered sarcoplasmic reticulum Ca2+-ATPase gene expression in the human ventricle during end-stage heart failure. J. Clin. Invest. 85, 305–309 (1990).
Morgan, J. P. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N. Engl. J. Med. 325, 625–632 (1991).
Lindner, M., Erdmann, E. & Beuckelmann, D. J. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J. Mol. Cell Cardiol. 30, 743–749 (1998).
Campbell, A. M., Kessler, P. D. & Fambrough, D. M. The alternative carboxyl termini of avian cardiac and brain sarcoplasmic reticulum/endoplasmic reticulum Ca2+-ATPases are on opposite sides of the membrane. J. Biol. Chem. 267, 9321–9325 (1992).
Verboomen, H., Wuytack, F., Van den Bosch, L., Mertens, L. & Casteels, R. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca2+-transport ATPase (SERCA2a/b). Biochem. J. 303, 979–984 (1994).
Verboomen, H., Wuytack, F., De Smedt, H., Himpens, B. & Casteels, R. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem. J. 286, 591–595 (1992).
Gélébart, P., Martin, V., Enouf, J. & Papp, B. Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem. Biophys. Res. Commun. 303, 676–684 (2003).
Dally, S. et al. Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c). Biochem. J. 395, 249–258 (2006).
Arai, M., Matsui, H. & Periasamy, M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ. Res. 74, 555–564 (1994).
Feldman, A. M., Weinberg, E. O., Ray, P. E. & Lorell, B. H. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ. Res. 73, 184–192 (1993).
Kiss, E., Ball, N. A., Kranias, E. G. & Walsh, R. A. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca2+-ATPase protein levels. Effects on Ca2+ transport and mechanics in compensated pressure-overload hypertrophy and congestive heart failure. Circ. Res. 77, 759–764 (1995).
Zarain-Herzberg, A., Rupp, H., Elimban, V. & Dhalla, N. S. Modification of sarcoplasmic reticulum gene expression in pressure overload cardiac hypertrophy by etomoxir. FASEB J. 10, 1303–1309 (1996).
Zarain-Herzberg, A., Afzal, N., Elimban, V. & Dhalla, N. S. Decreased expression of cardiac sarcoplasmic reticulum Ca2+-pump ATPase in congestive heart failure due to myocardial infarction. Mol. Cell Biochem. 163–164, 285–290 (1996).
Guo, X., Chapman, D. & Dhalla, N. S. Partial prevention of changes in SR gene expression in congestive heart failure due to myocardial infarction by enalapril or losartan. Mol. Cell Biochem. 254, 163–172 (2003).
Arai, M., Alpert, N. R., MacLennan, D. H., Barton, P. & Periasamy, M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ. Res. 72, 463–469 (1993).
Hasenfuss, G. et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ. Res. 75, 434–442 (1994).
Koss, K. L. & Kranias, E. G. Phospholamban: a prominent regulator of myocardial contractility. Circ. Res. 79, 1059–1063 (1996).
Brittsan, A. G. & Kranias, E. G. Phospholamban and cardiac contractile function. J. Mol. Cell Cardiol. 32, 2131–2139 (2000).
Movsesian, M. A., Karimi, M., Green, K. & Jones, L. R. Ca2+-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation 90, 653–657 (1994).
Schwinger, R. H. et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92, 3220–3228 (1995).
Linck, B. et al. Messenger RNA expression and immunological quantification of phospholamban and SR-Ca2+-ATPase in failing and nonfailing human hearts. Cardiovasc. Res. 31, 625–632 (1996).
Flesch, M. et al. Sarcoplasmic reticulum Ca2+ ATPase and phospholamban mRNA and protein levels in end-stage heart failure due to ischemic or dilated cardiomyopathy. J. Mol. Med. (Berl.) 74, 321–332 (1996).
Meyer, M. et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92, 778–784 (1995).
Kranias, E. G. & Hajjar, R. J. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660 (2012).
Sande, J. B. et al. Reduced level of serine 16 phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc. Res. 53, 382–391 (2002).
Netticadan, T., Temsah, R. M., Kawabata, K. & Dhalla, N. S. Sarcoplasmic reticulum Ca2+/Calmodulin-dependent protein kinase is altered in heart failure. Circ. Res. 86, 596–605 (2000).
Mishra, S., Sabbah, H. N., Jain, J. C. & Gupta, R. C. Reduced Ca2+-calmodulin-dependent protein kinase activity and expression in LV myocardium of dogs with heart failure. Am. J. Physiol. Heart Circ. Physiol. 284, H876–H883 (2003).
Huang, B., Wang, S., Qin, D., Boutjdir, M. & El-Sherif, N. Diminished basal phosphorylation level of phospholamban in the postinfarction remodeled rat ventricle: role of β-adrenergic pathway, Gi protein, phosphodiesterase, and phosphatases. Circ. Res. 85, 848–855 (1999).
Schwinger, R. H. et al. Reduced Ca2+-sensitivity of SERCA2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell Cardiol. 31, 479–491 (1999).
Nicolaou, P., Hajjar, R. J. & Kranias, E. G. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J. Mol. Cell Cardiol. 47, 365–371 (2009).
Yamada, M. et al. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 20, 1197–1199 (2006).
Medeiros, A. et al. Mutations in the human phospholamban gene in patients with heart failure. Am. Heart J. 162, 1088.e1–1095.e1 (2011).
Schmitt, J. P. et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299, 1410–1413 (2003).
Haghighi, K. et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl Acad. Sci. USA 103, 1388–1393 (2006).
Haghighi, K. et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J. Clin. Invest. 111, 869–876 (2003).
Bers, D. M. & Weber, C. R. Na/Ca exchange function in intact ventricular myocytes. Ann. N. Y. Acad. Sci. 976, 500–512 (2002).
Studer, R. et al. Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ. Res. 75, 443–453 (1994).
Hasenfuss, G. et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99, 641–648 (1999).
Schwinger, R. H. et al. Reduced sodium pump α1, α3, and β1-isoform protein levels and Na+, K+-ATPase activity but unchanged Na+-Ca2+ exchanger protein levels in human heart failure. Circulation 99, 2105–2112 (1999).
Piper, C. et al. Is myocardial Na+/Ca2+ exchanger transcription a marker for different stages of myocardial dysfunction? Quantitative polymerase chain reaction of the messenger RNA in endomyocardial biopsies of patients with heart failure. J. Am. Coll. Cardiol. 36, 233–241 (2000).
Flesch, M. et al. Evidence for functional relevance of an enhanced expression of the Na+–Ca2+ exchanger in failing human myocardium. Circulation 94, 992–1002 (1996).
Reinecke, H., Studer, R., Vetter, R., Holtz, I. & Drexler, H. Cardiac Na+/Ca2+ exchange activity in patients with end-stage heart failure. Cardiovasc. Res. 31, 48–54 (1996).
Piacentino, V. 3rd et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 92, 651–658 (2003).
Weber, C. R., Piacentino, V. 3rd, Houser, S. R. & Bers, D. M. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation 108, 2224–2229 (2003).
Bers, D. M., Eisner, D. A. & Valdivia, H. H. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ. Res. 93, 487–490 (2003).
Györke, S., Stevens, S. C. & Terentyev, D. Cardiac calsequestrin: quest inside the SR. J. Physiol. 587 (Pt 13), 3091–3094 (2009).
Györke, S. & Terentyev, D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77, 245–255 (2008).
Knollmann, B. C. New roles of calsequestrin and triadin in cardiac muscle. J. Physiol. 587, 3081–3087 (2009).
Kontula, K. et al. Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights. Cardiovasc. Res. 67, 379–387 (2005).
Postma, A. V. et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 91, e21–e26 (2002).
Lahat, H. et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 69, 1378–1384 (2001).
Song, L. et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 117, 1814–1823 (2007).
Kiarash, A. et al. Defective glycosylation of calsequestrin in heart failure. Cardiovasc. Res. 63, 264–272 (2004).
Houle, T. D., Ram, M. L., McMurray, W. J. & Cala, S. E. Different endoplasmic reticulum trafficking and processing pathways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp. Cell Res. 312, 4150–4161 (2006).
McFarland, T. P., Milstein, M. L. & Cala, S. E. Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J. Mol. Cell Cardiol. 49, 556–564 (2010).
Zhang, L., Kelley, J., Schmeisser, G., Kobayashi, Y. M. & Jones, L. R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272, 23389–23397 (1997).
Gergs, U. et al. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am. J. Physiol. Heart Circ. Physiol. 293, H728–H734 (2007).
Terentyev, D. et al. Triadin overexpression stimulates excitation–contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ. Res. 96, 651–658 (2005).
Engelhardt, S. et al. Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the β1-adrenergic receptor. FASEB J. 15, 2718–2720 (2001).
Pritchard, T. J. & Kranias, E. G. Junctin and the histidine-rich Ca2+ binding protein: potential roles in heart failure and arrhythmogenesis. J. Physiol. 587, 3125–3133 (2009).
Fan, G. C., Gregory, K. N., Zhao, W., Park, W. J. & Kranias, E. G. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am. J. Physiol. Heart Circ. Physiol. 287, H1705–H1711 (2004).
Arvanitis, D. A. et al. The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur. Heart J. 29, 2514–2525 (2008).
Kiewitz, R., Lyons, G. E., Schafer, B. W. & Heizmann, C. W. Transcriptional regulation of S100A1 and expression during mouse heart development. Biochim. Biophys. Acta 1498, 207–219 (2000).
Volkers, M. et al. S100A1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium 41, 135–143 (2007).
Remppis, A. et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. Biochim. Biophys. Acta 1313, 253–257 (1996).
Most, P. et al. Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J. Biol. Chem. 278, 33809–33817 (2003).
Kettlewell, S., Most, P., Currie, S., Koch, W. J. & Smith, G. L. S100A1 increases the gain of excitation–contraction coupling in isolated rabbit ventricular cardiomyocytes. J. Mol. Cell Cardiol. 39, 900–910 (2005).
Du, X. J. et al. Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol. Cell. Biol. 22, 2821–2829 (2002).
Boerries, M. et al. Ca2+-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol. Cell Biol. 27, 4365–4373 (2007).
Wehrens, X. H. & Marks, A. R. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem. Sci. 28, 671–678 (2003).
Kushnir, A. & Marks, A. R. The ryanodine receptor in cardiac physiology and disease. Adv. Pharmacol. 59, 1–30 (2010).
Avila, G., O'Connell, K. M., Groom, L. A. & Dirksen, R. T. Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J. Biol. Chem. 276, 17732–17738 (2001).
Lanner, J. T., Georgiou, D. K., Joshi, A. D. & Hamilton, S. L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2, a003996 (2010).
Song, D. W., Lee, J. G., Youn, H. S., Eom, S. H. & Kim do, H. Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Prog. Biophys. Mol. Biol. 105, 145–161 (2011).
Balshaw, D. M., Xu, L., Yamaguchi, N., Pasek, D. A. & Meissner, G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 276, 20144–20153 (2001).
Zhang, T. et al. The δC isoform of CAMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 92, 912–919 (2003).
Sharma, M. R., Jeyakumar, L. H., Fleischer, S. & Wagenknecht, T. Three-dimensional visualization of FKBP12.6 binding to an open conformation of cardiac ryanodine receptor. Biophys. J. 90, 164–172 (2006).
Xiao, B. et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem. J. 396, 7–16 (2006).
Marx, S. O. et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J. Cell Biol. 153, 699–708 (2001).
Lee, E. H. et al. N-terminal region of FKBP12 is essential for binding to the skeletal ryanodine receptor. J. Biol. Chem. 279, 26481–26488 (2004).
Shan, J. et al. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J. Clin. Invest. 120, 4375–4387 (2010).
Shannon, T. R. & Lew, W. Y. Diastolic release of calcium from the sarcoplasmic reticulum: a potential target for treating triggered arrhythmias and heart failure. J. Am. Coll. Cardiol. 53, 2006–2008 (2009).
Go, L. O. et al. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J. Clin. Invest. 95, 888–894 (1995).
Sainte Beuve, C. et al. Cardiac calcium release channel (ryanodine receptor) in control and cardiomyopathic human hearts: mRNA and protein contents are differentially regulated. J. Mol. Cell Cardiol. 29, 1237–1246 (1997).
Hain, J., Onoue, H., Mayrleitner, M., Fleischer, S. & Schindler, H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J. Biol. Chem. 270, 2074–2081 (1995).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Valdivia, H. H., Kaplan, J. H., Ellis-Davies, G. C. & Lederer, W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science 267, 1997–2000 (1995).
Xiao, B. et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ. Res. 96, 847–855 (2005).
Reiken, S. et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J. Cell Biol. 160, 919–928 (2003).
Yano, M. et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation 102, 2131–2136 (2000).
Ono, K. et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca2+ release in heart failure. Cardiovasc. Res. 48, 323–331 (2000).
Priori, S. G. et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001).
Cerrone, M. et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ. Res. 96, e77–e82 (2005).
Nicol, R. L., Frey, N. & Olson, E. N. From the sarcomere to the nucleus: role of genetics and signaling in structural heart disease. Annu. Rev. Genomics Hum. Genet. 1, 179–223 (2000).
Li, H., Rao, A. & Hogan, P. G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21, 91–103 (2010).
Molkentin, J. D. Calcineurin and beyond: cardiac hypertrophic signaling. Circ. Res. 87, 731–738 (2000).
Molkentin, J. D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63, 467–475 (2004).
Molkentin, J. D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Molkentin, J. D. & Olson, E. N. GATA4: a novel transcriptional regulator of cardiac hypertrophy? Circulation 96, 3833–3835 (1997).
Lim, H. W. et al. Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition. J. Mol. Cell Cardiol. 32, 697–709 (2000).
Frey, N. & Olson, E. N. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65, 45–79 (2003).
Meguro, T. et al. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ. Res. 84, 735–740 (1999).
Sussman, M. A. et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281, 1690–1693 (1998).
Ding, B. et al. Pressure overload induces severe hypertrophy in mice treated with cyclosporine, an inhibitor of calcineurin. Circ. Res. 84, 729–734 (1999).
Luo, Z., Shyu, K. G., Gualberto, A. & Walsh, K. Calcineurin inhibitors and cardiac hypertrophy. Nat. Med. 4, 1092–1093 (1998).
Anderson, M. E., Brown, J. H. & Bers, D. M. CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell Cardiol. 51, 468–473 (2011).
Hoch, B., Meyer, R., Hetzer, R., Krause, E. G. & Karczewski, P. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 84, 713–721 (1999).
Kirchhefer, U., Schmitz, W., Scholz, H. & Neumann, J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc. Res. 42, 254–261 (1999).
Maier, L. S. et al. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 92, 904–911 (2003).
Zhang, R. et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 11, 409–417 (2005).
Wu, Y. et al. Suppression of dynamic Ca2+ transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J. Mol. Cell Cardiol. 40, 213–223 (2006).
Li, J. et al. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ. Res. 99, 1092–1099 (2006).
Grueter, C. E., Colbran, R. J. & Anderson, M. E. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J. Mol. Med. (Berl.) 85, 5–14 (2007).
Bartel, S. et al. Phosphorylation of phospholamban at threonine-17 in the absence and presence of β-adrenergic stimulation in neonatal rat cardiomyocytes. J. Mol. Cell Cardiol. 32, 2173–2185 (2000).
Hagemann, D. & Xiao, R. P. Dual site phospholamban phosphorylation and its physiological relevance in the heart. Trends Cardiovasc. Med. 12, 51–56 (2002).
Mattiazzi, A., Mundiña-Weilenmann, C., Guoxiang, C., Vittone, L. & Kranias, E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc. Res. 68, 366–375 (2005).
Brixius, K., Wollmer, A., Bölck, B., Mehlhorn, U. & Schwinger, R. H. Ser16- but not Thr17-phosphorylation of phospholamban influences frequency-dependent force generation in human myocardium. Pflugers Arch. 447, 150–157 (2003).
Hagemann, D. et al. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J. Biol. Chem. 275, 22532–22536 (2000).
Ji, Y. et al. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J. Biol. Chem. 278, 25063–25071 (2003).
Wehrens, X. H. et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304, 292–296 (2004).
Li, L., Satoh, H., Ginsburg, K. S. & Bers, D. M. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J. Physiol. 501, 17–31 (1997).
Maier, L. S. et al. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 92, 904–911 (2003).
Dzhura, I., Wu, Y., Colbran, R. J., Balser, J. R. & Anderson, M. E. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2, 173–177 (2000).
Grueter, C. E. et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the β subunit by CaMKII. Mol. Cell 23, 641–650 (2006).
Wu, Y. et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106, 1288–1293 (2002).
Muller, O. J. et al. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc. Res. 59, 380–389 (2003).
del Monte, F. et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 100, 2308–2311 (1999).
Prunier, F. et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation 118, 614–624 (2008).
Kawase, Y. et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 51, 1112–1119 (2008).
del Monte, F. et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation 104, 1424–1429 (2001).
Sakata, S. et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J. Mol. Cell Cardiol. 42, 852–861 (2007).
Miyamoto, M. I. et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. USA 97, 793–798 (2000).
del Monte, F. et al. Transcriptional changes following restoration of SERCA2a levels in failing rat hearts. FASEB J. 18, 1474–1476 (2004).
Nayak, S. & Herzog, R. W. Progress and prospects: immune responses to viral vectors. Gene Ther. 17, 295–304 (2010).
Asokan, A., Schaffer, D. V. & Samulski, R. J. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 20, 699–708 (2012).
Mariani, J. A. et al. Augmentation of left ventricular mechanics by recirculation-mediated AAV2/1-SERCA2a gene delivery in experimental heart failure. Eur. J. Heart Fail. 13, 247–253 (2011).
del Monte, F. et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc. Natl Acad. Sci. USA 101, 5622–5627 (2004).
Cutler, M. J., Wan, X., Laurita, K. R., Hajjar, R. J. & Rosenbaum, D. S. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ. Arrhythm. Electrophysiol. 2, 686–694 (2009).
Lyon, A. R. et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ. Arrhythm. Electrophysiol. 4, 362–372 (2011).
Chen, Y. et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation 109, 1898–1903 (2004).
Davia, K. et al. SERCA2a overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J. Mol. Cell. Cardiol. 33, 1005–1015 (2001).
Jaski, B. E. et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 15, 171–181 (2009).
Jessup, M. et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313 (2011).
Eizema, K. et al. Adenovirus-based phospholamban antisense expression as a novel approach to improve cardiac contractile dysfunction: comparison of a constitutive viral versus an endothelin-1-responsive cardiac promoter. Circulation 101, 2193–2199 (2000).
Hoshijima, M. et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 8, 864–871 (2002).
Dieterle, T. et al. Gene transfer of a phospholamban-targeted antibody improves calcium handling and cardiac function in heart failure. Cardiovasc. Res. 67, 678–688 (2005).
Watanabe, A. et al. Phospholamban ablation by RNA interference increases Ca2+ uptake into rat cardiac myocyte sarcoplasmic reticulum. J. Mol. Cell Cardiol. 37, 691–698 (2004).
Suckau, L. et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation 119, 1241–1252 (2009).
Neumann, J. et al. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am. J. Physiol. 265, H257–H266 (1993).
Hubbard, M. J. & Cohen, P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci. 18, 172–177 (1993).
Steenaart, N. A., Ganim, J. R., Di Salvo, J. & Kranias, E. G. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch. Biochem. Biophys. 293, 17–24 (1992).
Neumann, J. et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell Cardiol. 29, 265–272 (1997).
Gupta, R. C. et al. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 285, H2373–H2381 (2003).
Nicolaou, P., Hajjar, R. J. & Kranias, E. G. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J. Mol. Cell Cardiol. 47, 365–371 (2009).
Carr, A. N. et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell Biol. 22, 4124–4135 (2002).
El-Armouche, A. et al. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in β-adrenergic signaling. FASEB J. 17, 437–439 (2003).
El-Armouche, A., Pamminger, T., Ditz, D., Zolk, O. & Eschenhagen, T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc. Res. 61, 87–93 (2004).
Pathak, A. et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ. Res. 96, 756–766 (2005).
Nicolaou, P. et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ. Res. 104, 1012–1020 (2009).
Wittköpper, K. et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J. Clin. Invest. 120, 617–626 (2010).
Kawashima, H. et al. Protein phosphatase inhibitor-1 augments a protein kinase A-dependent increase in the Ca2+ loading of the sarcoplasmic reticulum without changing its Ca2+ release. Circ. J. 73, 1133–1140 (2009).
Geiss-Friedlander, R. & Melchior, F. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 (2007).
Woo, C. H. & Abe, J. SUMO—a post-translational modification with therapeutic potential? Curr. Opin. Pharmacol. 10, 146–155 (2010).
Jakobs, P. M. et al. Novel lamin A/C mutations in two families with dilated cardiomyopathy and conduction system disease. J. Card. Fail. 7, 249–256 (2001).
Zhang, Y. Q. & Sarge, K. D. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J. Cell Biol. 182, 35–39 (2008).
Kho, C. et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 (2011).
Wehrens, X. H. et al. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc. Natl Acad. Sci. USA 103, 511–518 (2006).
Belevych, A. E. et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc. Res. 90, 493–502 (2011).
Loughrey, C. M. et al. Over-expression of FK506-binding protein FKBP12.6 alters excitation–contraction coupling in adult rabbit cardiomyocytes. J. Physiol. 556, 919–934 (2004).
Huang, F., Shan, J., Reiken, S., Wehrens, X. H. & Marks, A. R. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc. Natl Acad. Sci. USA 103, 3456–3461 (2006).
Xu, L., Tripathy, A., Pasek, D. A. & Meissner, G. Potential for pharmacology of ryanodine receptor/calcium release channels. Ann. N. Y. Acad. Sci. 853, 130–148 (1998).
McCauley, M. D. & Wehrens, X. H. Targeting ryanodine receptors for anti-arrhythmic therapy. Acta Pharmacol. Sin. 32, 749–757 (2011).
Kaneko, N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev. Res. 33, 429–438 (1994).
Hunt, D. J. et al. K201 (JTV519) suppresses spontaneous Ca2+ release and 3H ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem. J. 404, 431–438 (2007).
Inagaki, K., Kihara, Y., Izumi, T. & Sasayama, S. The cardioprotective effects of a new 1,4-benzothiazepine derivative, JTV519, on ischemia/reperfusion-induced Ca2+ overload in isolated rat hearts. Cardiovasc. Drugs Ther. 14, 489–495 (2000).
Kumagai, K., Nakashima, H., Gondo, N. & Saku, K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 14, 880–884 (2003).
Kohno, M. et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am. J. Physiol. Heart Circ. Physiol. 284, H1035–H1042 (2003).
Ito, K. et al. JTV-519, a novel cardioprotective agent, improves the contractile recovery after ischaemia-reperfusion in coronary perfused guinea-pig ventricular muscles. Br. J. Pharmacol. 130, 767–776 (2000).
Inagaki, K. et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of δ-isoform of protein kinase C in rat ventricular myocardium. Circulation 101, 797–804 (2000).
Toischer, K. et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res. Cardiol. 105, 279–287 (2010).
Liu, N. et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 99, 292–298 (2006).
Bellinger, A. M. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15, 325–330 (2009).
Fauconnier, J. et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA 107, 1559–1564 (2010).
International Standard Randomised Controlled Trial Number Register. Controlled-trials.com [online] (2012).
Currie, S., Elliott, E. B., Smith, G. L. & Loughrey, C. M. Two candidates at the heart of dysfunction: the ryanodine receptor and calcium/calmodulin protein kinase II as potential targets for therapeutic intervention—an in vivo perspective. Pharmacol. Ther. 131, 204–220 (2011).
Sossalla, S. et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 107, 1150–1161 (2010).
Li, G., Hidaka, H. & Wollheim, C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol. Pharmacol. 42, 489–488 (1992).
Hvalby, O. et al. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc. Natl Acad. Sci. USA 91, 4761–4765 (1994).
Remppis, A. et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. Biochim. Biophys. Acta 1313, 253–257 (1996).
Most, P. et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J. Clin. Invest. 114, 1550–1563 (2004).
Most, P. et al. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation 114, 1258–1268 (2006).
Pleger, S. T. et al. Stable myocardial-specific AAV6–S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 115, 2506–2515 (2007).
Brinks, H. et al. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J. Am. Coll. Cardiol. 58, 966–973 (2011).
Pleger, S. T. et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 3, 92ra64 (2011).
Yano, M. et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107, 477–484 (2003).
Wehrens, X. H. et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function. Proc. Natl Acad. Sci. USA 102, 9607–9612 (2005).
Acknowledgements
This study was supported by National Institutes of Health Grants HL100396 and NIH/NHLBI Contract HHSN268201000045C.
Author information
Authors and Affiliations
Contributions
All authors researched the data and wrote the article. R. J. Hajjar reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. J. Hajjar is a stock-holder or director of Celladon and holds or has applied for a patent (Patent US8133878, Methods of Treating Restenosis) with the company. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kho, C., Lee, A. & Hajjar, R. Altered sarcoplasmic reticulum calcium cycling—targets for heart failure therapy. Nat Rev Cardiol 9, 717–733 (2012). https://doi.org/10.1038/nrcardio.2012.145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2012.145
This article is cited by
-
Venoarterial-extra corporeal membrane oxygenation-assisted parathyroidectomy for hypercalcemic crisis due to parathyroid carcinoma complicated by severe circulatory and respiratory failure: a case report
JA Clinical Reports (2023)
-
The RalGAPα1–RalA signal module protects cardiac function through regulating calcium homeostasis
Nature Communications (2022)
-
Calpains as novel players in the molecular pathogenesis of spinocerebellar ataxia type 17
Cellular and Molecular Life Sciences (2022)
-
RyR1-targeted drug discovery pipeline integrating FRET-based high-throughput screening and human myofiber dynamic Ca2+ assays
Scientific Reports (2020)
-
B-type natriuretic peptide and its role in altering Ca2+-regulatory proteins in heart failure—mechanistic insights
Heart Failure Reviews (2020)