Abstract
Background
Dapagliflozin reduces hyperglycaemia in patients with type 2 diabetes mellitus (T2DM) by increasing urinary glucose excretion.
Objectives
This study determined the overall safety profile of dapagliflozin in T2DM.
Methods
Safety of dapagliflozin in pooled analyses of phase IIb/III studies was evaluated. Patients received comparator or dapagliflozin as monotherapy, add-on to antidiabetic therapy, or as initial combination with metformin. Proportions of patients with adverse events (AEs) and prespecified parameters related to previous clinical observations and dapagliflozin’s action were assessed. The principal analysis used data from 12 placebo-controlled studies. Rare events were assessed across phase IIb/III studies, including special populations, comparator-controlled trials and ongoing long-term extensions.
Results
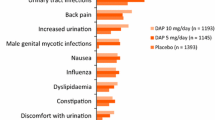
In placebo-controlled studies, hypoglycaemia was more common with dapagliflozin (11.8 %) than placebo (7.0 %), with imbalance driven by add-on of dapagliflozin to sulfonylurea or insulin. Urinary tract infections (4.8 vs 3.7 %), vulvovaginitis/balanitis and related infections (5.1 vs 0.9 %), and non-serious volume-related events (0.8 vs 0.4 %) occurred more often with dapagliflozin than placebo. No substantial AEs were seen on electrolytes or renal function. Pyelonephritis was rare and balanced among treatments; there were no imbalances in fractures or liver test elevations. Overall incidence of malignancies was balanced between groups. The incidence rate ratios of malignancy in certain organ systems were slightly lower for dapagliflozin (renal tract, female reproductive) and in others were slightly lower for control (breast, prostate, bladder). Most AEs associated with dapagliflozin were mild/moderate and related to the mechanism of action.
Conclusion
Dapagliflozin has a favourable and predictable tolerability profile, with reported events related to its mechanism of action.


Similar content being viewed by others
References
Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–24.
Woo V, Tang W, Salsali A, et al. Long-term efficacy of dapagliflozin monotherapy in patients with type 2 diabetes mellitus. Presented at International Diabetes Federation World Diabetes Congress; 2011 Dec 4–8; Dubai. Abstract no. D-0991.
Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–33.
Nauck MA, Del PS, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015–22.
Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13(10):928–38.
Wilding JP, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405–15.
Rosenstock J, Vico M, Wei L, et al. Dapagliflozin added-on to pioglitazone reduces HbA1c and mitigates weight grain with low incidence of hypoglycemia in type 2 diabetes [abstract]. Diabetes. 2011;60(Suppl 1):A84 (Abstract no. 986-P).
Henry RR, Murray AV, Marmolejo MH, et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66(5):446–56.
Kasichayanula S, Liu X, Shyu WC, et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel SGLT2 inhibitor, and metformin, pioglitazone, glimepiride, or sitagliptin in healthy subjects. Diabetes Obes Metab. 2011;13(1):47–54.
Kasichayanula S, Chang M, Liu X, et al. Lack of pharmacokinetic interactions between dapagliflozin and simvastatin, valsartan, warfarin, or digoxin. Adv Ther. 2012;29(2):163–77.
Kasichayanula S, Liu X, Griffen SC, et al. Effects of rifampin and mefenamic acid on the pharmacokinetics and pharmacodynamics of dapagliflozin [abstract]. Diabetes. 2012;61(Suppl 1):A607.
Carlson GF, Tou CK, Parikh S, et al. Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther. 2011;2(3):123–32.
List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650–7.
Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473–8.
Ptaszynska A, Johnsson KM, Apanovitch AM, et al. Safety of dapagliflozin in clinical trials for T2DM [abstract]. Diabetes. 2012;61(Suppl):A258.
Bailey CJ, Iqbal N, T’joen C, et al. Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab. 2012;14(10):951–9.
Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31.
Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15(5):432–40.
Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–71.
Wilding JP, Norwood P, T’joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32(9):1656–62.
Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37(3):740–50.
Lambers Heerspink HJ, de ZD, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–62.
Mudaliar S, Henry RR, Boden G, et al. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther. 2014;16(3):137–44.
Whaley JM, Tirmenstein M, Reilly TP, et al. Targeting the kidney and glucose excretion with dapagliflozin: preclinical and clinical evidence for SGLT2 inhibition as a new option for treatment of type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2012;5:135–48.
Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–83.
Singh P, Thomson SC. Renal homeostasis and tubuloglomerular feedback. Curr Opin Nephrol Hypertens. 2010;19(1):59–64.
Ljunggren O, Bolinder J, Johansson L, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2012;14(11):990–9.
Kohan DE, Fioretto P, List J, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment [abstract]. J Am Soc Nephrol. 2011;Suppl:232A. Abstract no. TH-PO524.
US National Institutes of Health, National Cancer Institute. Surveillance, epidemiology and end results. Standardized incidence ratio and confidence limits. National Cancer Institute website. Available from http://seer.cancer.gov/seerstat/WebHelp/Standardized_Incidence_Ratio_and_Confidence_Limits.htm. Accessed 22 Nov 2010.
Acknowledgments
This study was supported by AstraZeneca and Bristol-Myers Squibb. The sponsors were involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Medical writing and editorial assistance was provided by Alexandra Silveira, PhD, of PPSI (a PAREXEL company) and funded by AstraZeneca and Bristol-Myers Squibb. Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012 and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012. Agata Ptaszynska supervised the study, analysed and interpreted data and wrote and revised the article. James F. List, Kristina M. Johnsson, Shamik J. Parikh and Tjerk W.A. de Bruin contributed to the study concept and design, analyzed and interpreted data and wrote and revised the article. Anne Marie Apanovitch contributed to the study concept and design, contributed to statistical verification of data, analyzed and interpreted data and wrote and revised the article.
Conflict of interest
Agata Ptaszynska, Anne Marie Apanovitch. and James F. List. are employees and shareholders of Bristol-Myers Squibb. Kristina M. Johnsson is an employee of AstraZeneca. Shamik J. Parikh and Tjerk W.A. de Bruin are employees and shareholders of AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ptaszynska, A., Johnsson, K.M., Parikh, S.J. et al. Safety Profile of Dapagliflozin for Type 2 Diabetes: Pooled Analysis of Clinical Studies for Overall Safety and Rare Events. Drug Saf 37, 815–829 (2014). https://doi.org/10.1007/s40264-014-0213-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0213-4