Abstract
Abstract
Management of type 2 diabetes mellitus (T2DM) is complex and challenging, particularly for clinicians working in primary care who are faced with many competing clinical priorities. The range of available T2DM treatments has diversified significantly in recent years, generating a busy and data-rich environment in which evidence is rapidly evolving. Sodium-glucose cotransporter-2 inhibitor (SGLT2i) agents are a relatively new class of oral glucose-lowering therapy that have been available in the UK for approximately 5 years. These agents reduce the reabsorption of glucose in the kidney and increase its excretion via the urine. Conflicting messages and opinions within the clinical community have led to misconceptions concerning the efficacy, safety and appropriate position of SGLT2i therapies within the T2DM treatment pathway. To help address some of these concerns and provide advice regarding the appropriate place of these medicines in clinical practice, the Improving Diabetes Steering Committee was formed. The Committee worked together to develop this review article, providing a summary of relevant data regarding the use of SGLT2i medicines and focusing on specific considerations for appropriate prescribing within the T2DM management pathway. In addition, a benefit/risk tool has been provided (see Fig. 3) that summarises many of the aspects discussed in this review. The tool aims to support clinicians in identifying the people most likely to benefit from SGLT2i treatments, as well as situations where caution may be required.
Funding
Napp Pharmaceuticals Limited.
Similar content being viewed by others
The Role of the Improving Diabetes Steering Committee
The Improving Diabetes Steering Committee comprises a panel of expert advisers from across primary and secondary care who meet with the objective of improving diabetes care. The Committee aims to ensure that UK prescribers of diabetes medicines have access to balanced and accurate information/evidence concerning oral type 2 diabetes mellitus (T2DM) medicines, with a specific focus on the sodium-glucose cotransporter-2 inhibitor (SGLT2i) class of treatments.
The group is committed to providing healthcare colleagues with clarity concerning the evidence base supporting SGLT2i agents, highlighting the relative benefits and risks of these therapies. Educational materials and publications provided by the panel aim to increase confidence and understanding regarding the appropriate place of these medicines within the current T2DM treatment paradigm.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
The SGLT2 Inhibitor Class of Medicines
Mechanism of Action
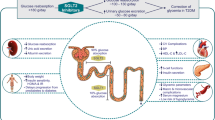
SGLT2 cotransporters are present in the early proximal convoluted tubule (PCT) of the kidney, where they actively reabsorb glucose to optimally maintain blood glucose levels [1]. SGLT2i medicines work independently of insulin to selectively inhibit reabsorption of glucose in the kidney, increasing excretion via the urine [1].
Three SGLT2i therapies are currently available for clinical use in the UK for the treatment of T2DM: canagliflozin (distributed in the UK by Napp Pharmaceuticals Limited), dapagliflozin (AstraZeneca UK Limited) and empagliflozin (Boehringer Ingelheim Limited) [2,3,4]. These therapies are all very similar in terms of their mechanisms of action, although canagliflozin is known to also have affinity for SGLT1 cotransporters found in the intestine and kidneys [2]. Phase 3 studies suggest that this property may contribute to the enhanced postprandial glucose-lowering action of canagliflozin 300 mg compared with canagliflozin 100 mg [2].
UK Treatment Guidelines
Based upon available efficacy and safety data, the National Institute for Health and Care Excellence (NICE) and the Scottish Intercollegiate Guideline Network (SIGN) recommend that SGLT2i therapies can be considered alongside other glucose-lowering medicines as an option at the first intensification of treatment for T2DM, following failure to achieve control with metformin, or as a first-line treatment in cases of metformin intolerance [5,6,7]. SGLT2i medicines may also be used as add-on third-line therapies. For example, they may be used in combination with other glucose-lowering agents such as oral therapies or glucagon-like peptide 1 receptor agonists (GLP-1 RAs) or with insulin [2,3,4,5,6]. Canagliflozin and empagliflozin may be prescribed in combination with pioglitazone, but dapagliflozin is not approved for combination treatment with pioglitazone [2,3,4].
Figures 1 and 2 present an overview of the NICE and SIGN treatment algorithms for blood glucose-lowering and T2DM management, respectively [5, 6]. Recommendations concerning SGLT2i treatments apply across the class of medicines, rather than to individual therapies or molecules [5, 6].
NICE treatment algorithm for blood glucose-lowering therapy in adults with T2DM [5]. © NICE (2015) NG28 Type 2 diabetes in adults: management [5]. Available from: http://www.nice.org.uk/guidance/ng28. All rights reserved. Subject to Notice of rights. Requests to reuse NICE content outside of the United Kingdom should be sent to nice@nice.org.uk. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. (Abbreviations: DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1 glucagon-like peptide-1, SGLT2i sodium-glucose cotransporter-2 inhibitors, SU sulfonylurea). Recommendations that cover DPP-4is, GLP-1 mimetics and SUs refer to these groups of drugs at a class level. aWhen prescribing pioglitazone, exercise particular caution if the person is at high risk of the AEs of the drug. Pioglitazone is associated with an increased risk of heart failure, bladder cancer and bone fracture. Known risk factors for these conditions, including increased age, should be carefully evaluated before treatment: see the manufacturers’ summaries of product characteristics for details. MHRA guidance (2011) advises that ‘prescribers should review the safety and efficacy of pioglitazone in individuals after 3–6 months of treatment to ensure that only those deriving benefit continue to be treated’. bSee NICE Technology Appraisal Guidance 288 and 418, 315 and 336 on dapagliflozin, canagliflozin and empagliflozin, respectively. All three SGLT-2 inhibitors are recommended as options in dual therapy regimens with metformin under certain conditions, as options in triple therapy regimens and in combination with insulin. All three are also options as monotherapies in adults in whom metformin is contraindicated or not tolerated. Serious and life-threatening cases of DKA have been reported in people taking SGLT2is (canagliflozin, dapagliflozin or empagliflozin) or shortly after stopping the SGLT2i. MHRA guidance (2015) advises testing for raised ketones in people with symptoms of DKA, even if plasma glucose levels are near normal. cOnly continue GLP-1 mimetic therapy if the person has a beneficial metabolic response [a reduction of HbA1c by at least 11 mmol/mol (1.0%) and a weight loss of at least 3% of initial body weight in 6 months]. dIf metformin is contraindicated or not tolerated, repaglinide is both clinically effective and cost effective in adults with type 2 diabetes. However, discuss with any person for whom repaglinide is being considered that there is no licensed non-metformin-based combination containing repaglinide that can be offered at first intensification. eDrugs in dual therapy should be introduced in a stepwise manner, checking for tolerability and effectiveness of each drug. fMHRA guidance (2011) notes that cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in individuals with risk factors for the development of cardiac failure. People should be observed for signs and symptoms of heart failure, weight gain, and oedema. Pioglitazone should be discontinued if any deterioration in cardiac status occurs. gThe recommendations in this guideline also apply to any current and future biosimilar product(s) of insulin glargine that have an appropriate Marketing Authorisation that allows the use of the biosimilar(s) in the same indication
SIGN treatment algorithm for T2DM management [6]. Algorithm summarises evidence from the guideline in the context of the clinical experience of the Guideline Development Group. It does not apply in severe renal or hepatic insufficiency. 1Consider dose reduction. 2Do not delay if first line options not tolerated/inappropriate. 3See guideline pages 23 and 26–27. 4See BNF: specific agents can be continued at reduced dose. 5See BNF: no dose reduction required for linagliptin. 6Pioglitazone is contraindicated in people with (or with a history of) heart failure or bladder cancer. 7Do not combine dapagliflozin with pioglitazone. 8Caution with exenatide when eGFR < 50 ml/min/1.73 m2. 9Adjust according to response. 10Driving, occupational hazards, risk of falls, previous history. Prescribers should refer to the British National Formulary (www.medicinescomplete.com), the Scottish Medicines Consortium (www.scottishmedicines.org.uk) and Medicines and Healthcare products Regulatory Agency (MHRA) warnings for updated guidance on licensed indications, full contraindications and monitoring requirements. *Continue medication at each stage if EITHER individualised target achieved OR HbA1c falls more than 0.5% (5.5 mmol/mol) in 3–6 months. Discontinue if evidence that it is ineffective. CKD 3A chronic kidney disease stage 3A (estimated glomerular filtration rate 45–59 ml/min/1.73 m2); CV cardiovascular
Efficacy Data
SGLT2i therapies have demonstrated robust efficacy outcomes concerning glycaemia, blood pressure, intrarenal hemodynamic properties, weight loss and albuminuria [8,9,10,11,12,13,14,15,16,17,18].
Reduction in Glycated Haemoglobin (HbA1c)
Each of the SGLT2i treatments available within the UK have proven efficacy as monotherapies and combination therapies [2–4]. Canagliflozin has demonstrated efficacy in nine randomised controlled trials (RCTs), involving 10,285 people with T2DM, with the 300 mg dosage of canagliflozin proving to be particularly efficacious in terms of glycaemic control [2, 10, 16]. Dapagliflozin has shown effective treatment outcomes in 14 RCTs, conducted with 7056 people with T2DM [3]. The efficacy of empagliflozin has been established in 12 RCTs involving 14,663 T2DM individuals, with the 25 mg dosage providing additional improvements in blood glucose control compared with the 10 mg dosage [4, 12].
In addition, a systematic review and network meta-analysis of RCT data (from January 2005 to January 2015) concerning SGLT2i efficacy, compared with placebo, demonstrated significant reductions in HbA1c with each treatment when prescribed as monotherapy and dual therapy, with few differences between the class members concerning outcomes [11]. Compared with placebo, reductions in HbA1c were greater with canagliflozin 300 mg monotherapy (-1.23%) than with other SGLT2i treatments or canagliflozin 100 mg [11]. Regarding dual therapy, canagliflozin 300 mg achieved the greatest reductions in HbA1c, although statistical significance was only observed in comparison to canagliflozin 100 mg [mean difference 0.15; confidence interval (CI) 0.04–0.26] [11].
Weight Loss
SGLT2i agents are associated with weight loss, much of which is due to a reduction in visceral fat, rather than urinary glucose excretion [8,9,10]. As a result, these treatments should be helpful for individuals aspiring to lower or control their body weight, an important factor and common feature of T2DM management [19].
Cardiovascular (CV) Efficacy
A growing wealth of evidence from large RCTs and observational studies demonstrates that SGLT2i treatments reduce the risk of serious CV complications, progression of kidney disease, and death in people at risk of major adverse cardiac events [12,13,14,15,16,17,18]. As a large proportion of people living with T2DM also have an increased risk of CV morbidities and mortality, these data are particularly relevant and may have implications for clinical practice [20].
A recent network meta-analysis including 236 trials with 176,310 participants (published before October 2017) has examined RCTs involving SGLT2is, GLP-1 RAs and dipeptidyl peptidase-4 inhibitors (DPP-4is) [21]. The analysis showed that SGLT2i treatments are associated with significantly reduced risk of all-cause mortality, heart failure (HF) and myocardial infarction (MI) versus controls (placebo or no treatment) [21]. The study also revealed that the SGLT2is and GLP-1 RAs were associated with significantly lower rates of all-cause mortality and CV events in published RCTs compared with DPP-4is [21].
In recognition of the evidence in this area, the SIGN 2017 guideline also recommends using SGLT2i therapies with proven CV benefit (currently empagliflozin and canagliflozin) for those individuals with T2DM and established CV disease (CVD) [6, 12, 16].
Key Published CV RCTs
Two large CV outcome trials, the EMPA-REG trial and the CANVAS Program, have provided robust data concerning CV endpoints [12,13,14,15,16,17,18]. These trials are considered by the Improving Diabetes Steering Committee to have an evidence level of ‘A’ under the American Diabetes Association (ADA) evidence-grading system (also used to support the benefit/risk tool, see Fig. 3) [22].
EMPA-REG OUTCOME Trial [12]
The EMPA-REG OUTCOME trial examined the efficacy and safety of empagliflozin in reducing CV mortality and morbidity in 7020 people with T2DM considered to be at a high risk of experiencing CV events [12]. Empagliflozin (10 or 25 mg) was administered in addition to standard care, and CV outcomes were compared against placebo. Median follow-up was 3.1 years and the primary composite outcome was a reduction in 3-point major adverse CV events (3P-MACE), comprising of death from CV causes, nonfatal MI or nonfatal stroke [12]. The key secondary outcome was a composite of primary endpoints and hospitalisation for unstable angina [12].
The EMPA-REG OUTCOME trial met its primary endpoint, demonstrating a 14% relative risk reduction (RRR) in 3P-MACE [hazard ratio (HR) 0.86; 95.02% CI 0.74–0.99; P = 0.04 for superiority]. Empagliflozin treatment was associated with significantly lower rates of CV death compared with placebo, with a RRR of 38% (HR 0.62; 95% CI 0.49–0.77; P < 0.001) [12]. However, the study demonstrated no significant difference concerning MI or stroke rates with empagliflozin treatment versus placebo [12]. In addition, compared with placebo, empagliflozin treatment resulted in a RRR of 35% for hospitalisations due to HF (HHF) (HR 0.65; 95% CI 0.50–0.85; P = 0.002) and a 32% RRR for all-cause mortality (HR 0.68; 95% CI 0.57–0.82, P < 0.001) [12]. Kidney function was maintained in the active treatment groups and the incidence of renal events, including acute renal failure and kidney injury, was lower in participants treated with empagliflozin [15].
A number of subsequent subanalyses have been published that further explored the EMPA-REG data. These demonstrated improvements concerning CV endpoints in individuals across the HHF risk spectrum and those with peripheral artery disease (PAD), and examined renal outcomes within this cohort [13,14,15].
The CANVAS Program [16]
The Canagliflozin Cardiovascular Assessment Study (CANVAS) Program comprised an integrated analysis of data from two trials: CANVAS and CANVAS-renal (CANVAS-R) [16]. CANVAS examined CV safety outcomes and CANVAS-R incorporated progression of albuminuria [23]. In total, 10,142 people with T2DM and at high CV risk were treated with canagliflozin (100 or 300 mg) or placebo, alongside standard T2DM care [16, 23]. Mean follow-up was 188.2 weeks [16, 23]. As with the EMPA-REG trial, the composite primary endpoint was 3P-MACE [16]. Secondary outcomes were death from any cause, CV death, progression of albuminuria, and the composite of death from CV causes and HHF [16].
The CANVAS trial met its primary endpoint, demonstrating a 14% RRR in 3P-MACE versus placebo (HR 0.86; 95% CI 0.75–0.97; P < 0.001 for noninferiority; P = 0.02 for superiority) [16]. Compared with placebo, a RRR of 27% was observed concerning progression to albuminuria with canagliflozin (HR 0.73; 95% CI 0.67–0.79). A reduction in the composite exploratory outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), need for renal replacement therapy, or death from renal causes, was also demonstrated with canagliflozin therapy (HR 0.60; 95% CI 0.47–0.77) [16].
Real-World CV OUTCOME Studies
The EASEL Study [17]
EASEL was a US observational study of 25,258 people with T2DM and established CVD within the US Department of Defence Military Health System [17]. The study compared outcomes in people receiving SGLT2i therapies with those of individuals prescribed non-SGLT2i treatments, and the median follow-up was 1.6 years [17]. The primary endpoint was a composite of all-cause mortality and HHF. In addition, MACE (comprising all-cause mortality, nonfatal MI and nonfatal stroke), a composite of MACE and HHF, and individual endpoints were assessed [17]. Of the 12,629 individuals receiving SGLT2i therapy, 58.1% were treated with canagliflozin, 26.5% were prescribed empagliflozin and 15.4% received dapagliflozin [17].
SGLT2i therapy was associated with lower rates of the primary composite endpoint, compared with non-SGLT2i treatment, with an incidence rate of 1.73 versus 3.01 events per 100 person-years (HR 0.57; 95% CI 0.50–0.65) [17]. The all-cause mortality rate was reduced in those receiving SGLT2i therapies compared with non-SGLT2i treatments: 1.29 versus 2.26 events per 100 person years (HR 0.57; 95% CI 0.49–0.66; P < 0.0001). The HHF rate was lower with SGLT2i therapy: 0.51 versus 0.90 events per 100 person years (HR 0.57, 95% CI 0.45–0.73; P < 0.0001) [17]. The incidence of MACE was also reduced in those treated with SGLT2i agents compared with non-SGLT2i treatments: 2.31 versus 3.45 events per 100 person years (HR 0.67; 95% CI 0.60–0.75) [17].
CVD-REAL [18]
CVD-REAL was an international observational study comparing rates of HHF and death in people receiving SGLT2i treatments with those of other glucose-lowering therapies [18]. Data were collated from 309,056 individuals newly initiated on T2DM therapies (154,528 in each group), using medical claims, primary care/hospital records, and national registries in the US, Norway, Denmark, Sweden, Germany and the UK [18]. Of the SGLT2i agents examined, canagliflozin accounted for 53%, dapagliflozin for 42% and empagliflozin for 5% [18].
SGLT2i treatments were associated with lower rates of HHF (pooled HR 0.61; 95% CI 0.51–0.73; P < 0.001), all-cause mortality (pooled HR 0.49; 95% CI 0.41–0.57; P < 0.001) and the composite outcome of death or HHF (pooled HR 0.54; 95% CI 0.48–0.60; P < 0.001) [18]. In addition, a recent subanalysis of the global CVD-REAL data demonstrated a reduction in risk of MI (HR 0.85; 95% CI 0.72–1.00; P = 0.05) and stroke (HR 0.83; 95% CI 0.71–0.97; P = 0.02) with SGLT2i treatments compared with other glucose-lowering therapies [24].
These results reaffirmed the outcomes of the EMPA-REG trial and CANVAS Program in real-world practice [12, 16, 18]. No significant heterogeneity was found concerning outcomes across country settings. Most people included in the analysis did not have established CVD and had a lower CV-risk profile than those included in the EMPA-REG and CANVAS trials, indicating that a broader range of low-risk people with T2DM may also benefit from the protective effects of SGLT2i treatments in terms of reduction in CV events and mortality [18].
Ongoing CV and Renal Studies in T2DM
DECLARE-TIMI 58 [25, 26]
In line with the current focus on CV outcomes, the ongoing Dapagliflozin Effect on CardiovascuLAR Events (DECLARE-TIMI 58) trial has been designed to examine dapagliflozin treatment in a high-risk T2DM population [25]. This multicentre RCT will examine treatment outcomes in 17,160 people with T2DM and CVD or multiple risk factors for CVD (e.g. dyslipidaemia, hypertension). Dapagliflozin (10 mg) treatment will be compared against placebo in participants receiving standard T2DM care [25].
The time to first event for the co-primary endpoint of 3P-MACE (CV death, nonfatal MI or nonfatal ischaemic stroke) and composite of time to CV death or HHF will be assessed.
Secondary composite outcomes will comprise time to first event of the renal endpoint: confirmed sustained ≥ 40% decrease in eGFR (to eGFR < 60 ml/min/1.73 m2 [2]) and/or end-stage kidney disease (ESKD) and/or renal or CV death. Time to all-cause mortality will also be assessed [25, 26].
CREDENCE [27, 28]
Although EMPA-REG and the CANVAS Program have examined some exploratory renal endpoints, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial is the first dedicated prospective renal RCT concerning SGLT2i treatment in T2DM [12,13,14,15,16, 27, 28]. This study is important for the SGLT2i class of medicines, as people with T2DM and kidney disease are at increased risk of CV events [29].
CREDENCE is a multicentre RCT comparing canagliflozin (100 mg) with placebo in people with T2DM and diabetic nephropathy who are receiving standard care, including the maximum tolerated daily dose of an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) [27, 28].
CREDENCE will assess the efficacy and safety of canagliflozin in preventing clinically important kidney and CV outcomes. The primary composite endpoint is time to ESKD, doubling of serum creatinine, and renal or CV death [27, 28].
Secondary endpoints include a composite of CV death and congestive heart failure (CHF) hospitalisation; a renal composite endpoint of ESKD and a doubling of serum creatinine; CV death and all-cause mortality; a CV composite of 3P-MACE, CHF hospitalisation and unstable angina hospitalisation. The study is expected to enrol approximately 4400 people with T2DM [27, 28].
Safety and Tolerability
As is the case with all medicines, clinical experience and emerging clinical data have revealed factors that may influence the safety profile of SGLT2i therapies, as well as highlighting certain populations who may be at increased risk of tolerability issues. The main areas in which clinicians may require further support/guidance concerning risk management are discussed below, alongside the relevant published evidence.
Genital Infections
Genital infections (e.g. thrush) are a common adverse event (AE) associated with SGLT2is [12, 16, 30–32]. However, practical hygiene advice for people with T2DM and their partners may help to prevent this issue. Topical treatments or appropriate oral treatments are helpful for managing mild to moderate infections [30, 31]. Urinary symptoms due to glucosuria can be an issue for people prescribed SGLT2i medicines [2,3,4]. However, urinary tract infections (UTIs) are relatively rare and can be addressed with standard oral antibiotic treatments [30,31,32].
These are manageable issues that usually occur early during treatment exposure and are typically self-limiting [30, 31]. It is important to manage people’s expectations at the start of treatment so that they are aware of these common side effects and are able to tolerate them better or seek medical support early before they become an issue.
Lower Limb Amputations (LLAs) and Bone Fractures
LLAs
Guidance issued by the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) has highlighted the need for caution regarding the use of SGLT2i treatments in those at high risk of LLAs [33]. The report was issued as a result of a safety signal raised during the CANVAS Program. Although LLA incidence was low overall, a significant difference was observed concerning the number of events that occurred in the treatment arm compared with the placebo arm (Table 1) [16].
The EMA PRAC report has suggested that, based on available reported data, a class effect across all SGLT2i treatments cannot be ruled out [33]. Evidence is conflicting in this area [16, 34]. Overall, the crude LLA incidence rate in the treatment arm was similar in the EMPA-REG and CANVAS trials: 0.65 per 100 person years and 0.63 per 100 person years, respectively (Table 1) [16, 34]. However, in the EMPA-REG trial, the LLA incidence was the same in the pooled analysis for both the treatment and placebo arms [34]. Although a higher number of LLA events were recorded in the EMPA-REG placebo arm than in the CANVAS trial placebo arm (Table 1), key differences regarding the design of these trials (e.g. study populations, exposure times, primary endpoint assessments) make direct comparisons impossible [16, 34].
The incidence of LLAs does not seem to be dose dependent and the absolute risk appears to be higher in people who have had a previous amputation [16, 33]. The CANVAS study was conducted in a relatively high-risk group, including individuals with PAD and a history of amputations, which may have increased the likelihood of LLAs occurring in this cohort [16].
Observational data from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) identified 66 SGLT2i-associated amputations among 9,217,555 AE reports filed from across the globe [35]. Of these, the majority included canagliflozin as a concomitant medication, and most were toe amputations [35]. However, data from the large-scale US Truven MarketScan database, including 119,567 people with T2DM who were receiving SGLT2i therapies, revealed that there was no increase in below-knee leg extremity (BKLE) amputations with canagliflozin compared with those receiving non-SGLT2i treatments (Table 2) [17]. In contrast with the high-risk CANVAS Program and EMPA-REG study populations, just 13% of this study cohort had established CVD at baseline. In addition, the BKLE incidence rate was comparable for those receiving empagliflozin and canagliflozin (incidence rate: 1.39 with empagliflozin versus 1.26 with canagliflozin per 1000 person years) [17].
It should also be noted that the number of LLA events was very low in RCTs and observational studies compared with the number of people with T2DM treated [12, 16, 17]. Global pharmacovigilance data support this viewpoint [36]. A recent analysis of World Health Organization (WHO) individual case safety reports (ICSRs), including spontaneously reported adverse drug reactions (ADRs), identified just 79 reports of LLAs associated with SGLT2is from a total of 369,543 reports concerning blood glucose-lowering agents. This analysis showed that canagliflozin and empagliflozin were associated with an increased proportional reporting ratio (PRR) for LLAs, and dapagliflozin demonstrated an increased PRR for toe amputations only. However, pharmacovigilance data such as these may be subject to limitations, as they are dependent on accurate ADR reporting.
Appropriate advice for healthcare professionals has been issued by the UK Medicines and Healthcare products Regulatory Agency (MHRA) [37]. As with all people with T2DM, to minimise the risk of LLAs, ongoing monitoring and advice regarding preventative footcare should be provided, and it may be advisable to avoid SGLT2i use in the individuals at greatest risk, such as those with active foot ulceration or previous amputation [2,3,4, 37].
Bone Fractures
Data are inconsistent concerning the risk of bone fractures associated with SGLT2i therapies. EMPA-REG data and dapagliflozin studies have not demonstrated any meaningful differences concerning bone mineral density (BMD) or fracture incidence compared with placebo [9, 12]. Although the CANVAS trial highlighted a slight increase in fracture incidence (15.4 canagliflozin versus 11.9 placebo participants per 1000 person years; HR 1.26; 95% CI 1.04–1.52), these outcomes were not replicated in the CANVAS-R population [16]. Fractures tended to occur early in treatment and may have been linked to volume depletion and exacerbated by falls associated with hypotension [38]. In addition, studies examining BMD in elderly people treated with canagliflozin show only reductions in hip bone density, which was not a site of fracture identified within the CANVAS population, and may be partly due to weight loss, as bone strength was retained [16, 39].
Diabetic Ketoacidosis (DKA)
In 2015, the US FDA issued a safety communication regarding the risk of DKA in people with T2DM treated with SGLT2i therapies, and a warning was added to the label for each medicine within the class [40]. This was subsequently supported by the EMA and the MHRA, although the EMA also stated that the benefits of these medicines continue to outweigh the risks in the treatment of T2DM [2,3,4, 40,41,42].
Cases of DKA in people treated with SGLT2is are fairly rare [41, 42]. Risk factors that should be considered include individuals who are relatively insulin deficient (e.g. people with late-onset autoimmune diabetes who have been misdiagnosed as having type 2 diabetes), sudden reductions in insulin dose, increased requirement for insulin (due to illness, surgery or alcohol abuse), and conditions that restrict food intake (particularly carbohydrate consumption) or can lead to severe dehydration [40,41,42]. People with T2DM should be advised of the increased DKA risk associated with heavy alcohol consumption [40, 41].
Key symptoms of DKA include nausea, vomiting, abdominal pain, generalised malaise and shortness of breath [40,41,42]. In people treated with SGLT2is, DKA may present atypically, with relatively normal glucose levels (euglycaemic ketoacidosis) [40]. The MHRA recommend checking for raised ketone levels when DKA symptoms are present, even if blood glucose levels are near normal [41]. Where DKA is diagnosed, SGLT2i therapy should be discontinued immediately [2,3,4, 40,41,42].
Acute Illness
In cases of acute illness or planned surgical procedures, SGLT2i medicines should be suspended immediately until the person has recovered [2,3,4]. SGLT2i therapy may resume following full recovery [2,3,4].
People with T2DM should follow the recommended sick day rules, which also apply to some other medicines that people with T2DM may take regularly (e.g. metformin), seeking medical advice if they are unsure of how to manage their medicines during this time [43, 44].
Considerations When Initiating SGLT2i Therapies at an Early or Late Stage in the T2DM Treatment Pathway
SGLT2i agents may provide an effective option for people with T2DM at many stages along the treatment paradigm. Due to the increasing volume of evidence relating to the efficacy and safety profile of SGLT2i therapies in people with T2DM and CVD, these medicines are generally considered to be a logical option for many people as first-line, second-line or third-line therapy [5,6,7, 11,12,13,14,15,16,17,18].
As previously discussed, overweight or obese people with T2DM may particularly benefit from these therapies [8, 9]. SGLT2is may be prescribed in combination with insulin, although caution should be used in those requiring an insulin dose reduction due to insulinopenia, as they may be predisposed to ketosis [2,3,4, 40, 42]. SGLT2is may also be used in combination with GLP-1 RAs [2,3,4]. However, there is less evidence available for the use of this therapy combination [2,3,4].
Regarding renal function, SGLT2i therapy may be prescribed for people with eGFR ≥ 60 ml/min/1.73 m2. In those currently treated with canagliflozin and empagliflozin, treatment may continue until eGFR reaches 45 ml/min/1.73 m2 [2, 3]. Dapagliflozin may not be prescribed for individuals with eGFR < 60 ml/min/1.73 m2 [4]. It is important to note that a recent eGFR measurement should be used in clinical decision making, rather than historical measurements.
Malignancy is an important consideration for people living with chronic conditions such as T2DM. Phase III dapagliflozin studies reported a small number of bladder cancer cases, but these data were considered inconclusive concerning a causal relationship [45, 46]. To date, the large-scale CANVAS and EMPA-REG CV outcome trials have reported no increase in cancer rates with canagliflozin or empagliflozin [12, 16]. A recent meta-analysis of SGLT2is concluded that current evidence does not indicate an increased overall risk of cancer with SGLT2i therapies [47]. The DECLARE trial is expected to provide further insights concerning long-term SGLT2i use in this area in the coming months [25, 26].
SGLT2i therapies should be used with caution in those with very high HbA1c levels (86 mmol/mol or 10%), frail/elderly people, those with cognitive impairment, people who have rapidly progressed to requiring insulin (within one year of diagnosis) and individuals with a low body mass index (BMI) [2,3,4]. SGLT2i therapies should not be prescribed for people with a history of DKA, pancreatic disease, suspected type 1 diabetes, genetic diabetes or pregnant/breastfeeding women (or those planning pregnancy) [2,3,4].
SGLT2i treatments are not currently recommended for use with loop diuretics [2,3,4]. However, this may change over time as the evidence base in high-risk CV populations, particularly those with HF, continues to evolve [27].
Summary and Practical Considerations
The SGLT2i class of medicines provides an efficacious and generally well-tolerated treatment option for the management of T2DM [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. A wealth of RCT and real-world evidence supports the use of these therapies in achieving and maintaining control of blood glucose [8,9,10,11,12,13,14,15,16,17,18]. People with T2DM can experience weight loss with SGLT2i agents, which encourages them to adhere to treatment [8, 9]. In addition, studies have also revealed the cardio- and renoprotective aspects of these medicines [12,13,14,15,16,17,18].
Education regarding the risks of minor AEs such as genital thrush should be provided when initiating SGLT2i treatment, in order to manage people’s expectations and help them tolerate early issues so that they may gain optimum benefit from these medicines.
There are conflicting data concerning the risk of LLAs and bone fractures with these medicines, which has led to the EMA issuing a PRAC report recommending caution when prescribing all SGLT2i therapies in high-risk individuals (e.g. PAD, previous amputations) [9, 12, 16, 33, 36]. Much debate is ongoing within the clinical community regarding whether this is a real effect of these medicines or a result of trial design and study population. However, the crude number of LLA events recorded in major CV outcome trials was relatively low when compared with the number of people who demonstrated improved outcomes, such as a reduced risk of HHF or all-cause death [16, 34]. SGLT2is provide an effective option for many people with T2DM, including those with CVD and people with no history of peripheral vascular issues or LLAs. Ongoing monitoring and preventative footcare advice should be provided for those receiving SGLT2i medicines [2,3,4, 37].
DKA cases are relatively rare in people treated with SGLT2i medicines [2,3,4, 41]. DKA monitoring should be implemented, in line with MHRA guidelines [41]. Key symptoms include nausea, vomiting, abdominal pain, generalised malaise and shortness of breath [41].
SGLT2is may be initiated for appropriate people with T2DM at any stage along the treatment pathway where evidence supports their use and comorbidities do not compromise safety [5, 6].
The Improving Diabetes Steering Committee has developed a benefit/risk tool, provided in Fig. 3, that offers a quick reference guide concerning the specific areas covered in this review. The tool aims to provide clarity regarding common areas of confusion in clinical practice associated with the risk of LLAs and bone fractures, late and early use of SGLT2i treatments within the T2DM pathway, and risk of DKA.
The types of people or clinical situations that are likely to be seen in practice are highlighted in a traffic light system, in terms of risk:
-
Low risk (green) A robust evidence-base supports SGLT2i prescribing in these situations.
-
Moderate risk (amber) Prescribe SGLT2i agents with caution (some evidence supporting a benefit in these circumstances).
-
High risk (red) Do not prescribe SGLT2is in these situations (due to a lack of evidence, high risk of AEs, or licence restrictions).
An evidence level has been assigned to each risk category, based on RCT and observational data, as well as NICE/SIGN guidelines and the licensed indication for each therapy within the SGLT2i class of medicines. The level of evidence has been scored according to the ADA Evidence-Grading System (summarised in Table 3) [22].
Benefit/risk tool. (Abbreviations: T2DM type 2 diabetes mellitus, SGLT2i sodium-glucose cotransporter-2 inhibitor, ADA American Diabetes Association, RCT randomised controlled trial, BMI body mass index, LLAs lower leg amputations, PAD peripheral arterial disease, CV cardiovascular, CVD cardiovascular disease, eGFR estimated glomerular filtration rate, UTIs urinary tract infections, DKA diabetic ketoacidosis, CKD chronic kidney disease). ¶ SGLT2i therapies should be prescribed with caution in people requiring a rapid reduction in insulin dose, due to insulinopenia, which may increase DKA risk [2,3,4]. *Decisions should be based upon recent eGFR measurement, rather than historical tests. ‡SGLT2i therapies may be initiated in people with eGFR [3] 60 mL/min/1.73m2. Individuals already treated with canagliflozin or empagliflozin who demonstrate renal decline may continue treatment until eGFR reaches < 45 mL/min/1.73m2. Dapagliflozin should be discontinued for those who demonstrate eGFR < 60 mL/min/1.73m2 [2,3,4]. Urinary symptoms, due to glucosuria, can be an issue for people prescribed SGLT2i medicines [2,3,4]. However, UTIs are relatively rare and these medicines may be prescribed for people with a history of UTIs. ◆Monitor HbA1c levels regularly and cease SGLT2is if elevated levels continue, following treatment initiation. **SGLT2i treatments are not currently recommended for use alongside loop diuretics. However, this may be subject to change as the evidence-base evolves. EMPA-REG and CANVAS CV outcome trials included subgroups of people with T2DM who were receiving loop diuretics and ongoing trials aim to evaluate co-prescribing of these agents [12, 16, 27]. †SGLT2i treatment should be suspended in individuals with acute illness until fully recovered [2,3,4, 41, 42]
The tool aims to support clinicians in selecting appropriate people with T2DM for SGLT2i therapies, in line with the current evidence base and guidelines.
References
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355–66.
Napp Pharmaceuticals Limited. Canagliflozin: summary of product characteristics. 2017. https://www.medicines.org.uk/emc/product/8855. Accessed April 2018.
AstraZeneca UK Limited. Dapagliflozin: summary of product characteristics. 2017. https://www.medicines.org.uk/emc/product/7607. Accessed April 2018.
Boehringer Ingelheim Limited. Empagliflozin: summary of product characteristics. 2018. https://www.medicines.org.uk/emc/product/5441. Accessed April 2018.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2017. https://www.nice.org.uk/guidance/ng28. Accessed April 2018.
Scottish Intercollegiate Guideline Network (SIGN). Pharmacological management of glycaemic control in people with type 2 diabetes. Edinburgh: SIGN '154' - as per SIGN permission guidelines; 2017. http://www.sign.ac.uk/assets/sign154.pdf. Accessed April 2018. Cited 15 Jun 2018.
National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes. Technology appraisal guidance. 2016. https://www.nice.org.uk/guidance/ta390. Accessed April 2018.
Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119–26.
Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31.
Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med. 2016;128(4):371–80.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6(2):e009417.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39(5):363–70.
Verma S, Mazer CD, Al-Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circ. 2018;137(4):405–7.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose co-transporter 2 inhibitor: results from the EASEL population-based cohort study. Circulation. 2018;137(14):1450–9.
Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study. Circulation. 2017;136:249–59.
Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68(6):682–91.
Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46.
Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes. JAMA. 2018;319(15):1580–91.
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2017;40(1):S1–2.
Neal B, Perkovic V, Mahaffey KW, et al. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS- R trials. Diabetes Obes Metab. 2017;19(7):926–35.
Kosiborod M, Birkeland KI, Cavender MA et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: Results from the CVD-REAL study. Diabetes Obes Metab. 2018. 20(8):1983–7. https://doi.org/10.1111/dom.13299.
Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018;20(5):1102–10 (Epub ahead of print).
Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the dapagliflozin effect on cardiovascular events (DECLARE)–TIMI 58 Trial. Am Heart J. 2018;200:83–9.
Jardine MJ, Mahaffey KW, Neal B, et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462–72.
Clinicaltrials.gov. Evaluation of the effects of canagliflozin on renal and cardiovascular outcomes in participants with diabetic nephropathy (CREDENCE). https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed April 2018.
Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–40.
Gadzhanova S, Pratt N, Roughead E. Use of SGLT2 inhibitors for diabetes and risk of infection: analysis using general practice records from the NPS MedicineWise MedicineInsight program. Diabetes Res Clin Pract. 2017;130:180–5.
Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103(3):373–81.
Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(3):348–55.
European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC) assessment report. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Assessment_Report_-_Variation/human/002656/WC500230546.pdf. Accessed April 2018.
Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(1):e4–5.
Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA adverse event reporting system. Lancet Diabetes Endocrinol. 2017;5(9):680–1.
Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531–4.
UK Medicines and Healthcare products Regulatory Agency. 2017. SGLT2 inhibitors: updated advice on increased risk of lower-limb amputation (mainly toes). https://www.gov.uk/drug-safety-update/sglt2-inhibitors-updated-advice-on-increased-risk-of-lower-limb-amputation-mainly-toes. Accessed April 2018.
Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157–66.
Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44–51.
FDA. Drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed April 2018.
UK Medicines and Healthcare Products Regulatory Agency. 2016. SGLT2 inhibitors: updated advice on the risk of diabetic ketoacidosis. https://www.gov.uk/drug-safety-update/sglt2-inhibitors-updated-advice-on-the-risk-of-diabetic-ketoacidosis. Accessed April 2018.
EMA. EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors-20/European_Commission_final_decision/WC500202393.pdf. Accessed April 2018.
Diabetes UK. Dealing with illness. https://www.diabetes.org.uk/guide-to-diabetes/life-with-diabetes/illness. Accessed April 2018.
National Institute for Health and Care Excellence. Clinical knowledge summary. Diabetes—type 2. August 2017. https://cks.nice.org.uk/diabetes-type-2#!scenarioclarification:7. Accessed April 2018.
Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578. https://doi.org/10.1155/2014/719578.
Ptaszynska A, Cohen SM, Messing EM, Reilly TP, Johnsson E, Johnsson K. Assessing bladder cancer risk in type 2 diabetes clinical trials: the dapagliflozin drug development program as a ‘case study’. Diabetes Ther. 2015;6(3):357–75.
Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. 2017;60(10):1862–72.
NHS Scotland. Polypharmacy guidance—realistic prescribing. 2018. http://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/04/Polypharmacy-Guidance-2018.pdf. Accessed June 2018.
Acknowledgements
Funding
The Improving Diabetes Steering Committee is supported by an educational grant from Napp Pharmaceuticals Limited. Napp has reviewed this document for factual accuracy only. Sponsorship and article processing charges for this study were funded by Napp Pharmaceuticals Limited. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Medical Writing
Medical writing services were provided on behalf of the Steering Committee by Rebecca Down at Copperfox Communications Limited and Firstlight PR. Support for this assistance was funded by Napp Pharmaceuticals Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Professor John Wilding has received funding for providing educational sessions and/or attending advisory boards from Astellas, AstraZeneca, Biologix, Boehringer Ingelheim, Janssen, Lilly, Merck, Napp Pharmaceuticals, Novo Nordisk, Orexigen, Sanofi and Takeda. He has received travel grants to attend conferences from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk and Sanofi. He has institutional research grant support or funding for clinical trials from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, Sanofi and Takeda. Dr. Kevin Fernando has received speaker honoraria from all current manufacturers of SGLT2 inhibitors. Nicola Milne has received funding for provision of educational sessions or attendance at advisory boards from AstraZeneca, BD, Boehringer Ingelheim, Janssen, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk, Roche, Sanofi and Takeda. She has received travel grants to attend conferences from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Novartis. Dr. Marc Evans has received research support from Novo Nordisk and Takeda. He has received speaker fees and honoraria from Boehringer Ingelheim, Janssen, MSD, Napp Pharmaceuticals, Novartis and Novo Nordisk. Dr. Amar Ali has received speaker honoraria from all current manufacturers of SGLT2 inhibitors. Professor Steve Bain has received research support from Healthcare and Research Wales (Welsh Government), and honoraria from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim, Napp Pharmaceuticals and Merck. He has ownership interest in Glycosmedia (diabetes online news service). Debbie Hicks has received speaker honoraria from Roche, Sanofi and Takeda. She has received speaker and advisory board honoraria from Abbott, AstraZeneca, BD, Boehringer Ingelheim, Janssen, Lilly, MSD, Mylan, Napp Pharmaceuticals and Novo Nordisk. Educational sponsorship has been provided by BD, Boehringer Ingelheim, Lilly, MSD, Mylan, Novo Nordisk, and conference registration and subsistence from BD, Lilly and Novo Nordisk. Debbie does not hold any shares in any pharmaceutical company. June James has received funding for provision of educational sessions, resources and attendance at advisory boards from AstraZeneca, BD, Boehringer Ingelheim, Janssen, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk and Sanofi. Philip Newland-Jones has conducted research studies funded by, served as advisor to and received lecture honoraria and travel support from AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Lifescan, MSD, Mylan, Napp Pharmaceuticals, Novo Nordisk, Sanofi, Servier and Takeda. Dr. Dipesh Patel has received educational grant funding and speaker or advisory related honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Napp Pharmaceuticals, Novo Nordisk and Sanofi. Dr. Adie Viljoen has conducted research studies funded by, served as advisor to and received lecture honoraria and travel support from Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk, Pfizer, Regeneron, Sanofi Aventis and Tosoh.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6621683.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wilding, J., Fernando, K., Milne, N. et al. SGLT2 Inhibitors in Type 2 Diabetes Management: Key Evidence and Implications for Clinical Practice. Diabetes Ther 9, 1757–1773 (2018). https://doi.org/10.1007/s13300-018-0471-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0471-8