Abstract
Introduction
This randomized, open-label, crossover study investigated potential drug-drug interactions between the sodium glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin and the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin. Empagliflozin is a potent and selective SGLT-2 inhibitor that lowers blood glucose levels by inhibiting renal glucose reabsorption, leading to an increase in urinary glucose excretion. Sitagliptin lowers blood glucose through an insulin-dependent mechanism of action.
Methods
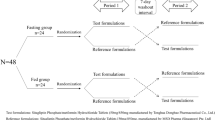
Sixteen healthy male volunteers received three treatments (A, B, C) in one of two treatment sequences (AB then C, or C then AB). In treatment AB, 50 mg empagliflozin was administered once daily (q.d.) for 5 days (treatment A), immediately followed by coadministration of 50 mg empagliflozin q.d. and 100 mg sitagliptin q.d. over 5 days (treatment B). In treatment C, 100 mg sitagliptin was administered q.d. for 5 days. A washout period of ≥7 days separated treatments AB and C.
Results
Coadministration of sitagliptin with empagliflozin did not have a clinically relevant effect on the area under the concentration-time curve of the analyte in plasma at steady state over a uniform dosing interval τ (AUCτ,ss) (geometric mean ratio [GMR] 110.4; 90% confidence interval [CI] 103.9, 117.3) or maximum measured concentration of the analyte in plasma at steady state over a uniform dosing interval τ (C max,ss) (GMR 107.6; 90% CI 97.0, 119.4) of empagliflozin. Coadministration of empagliflozin with sitagliptin did not have a clinically meaningful effect on the AUCτ,ss (GMR 103.1; 90% CI 98.9, 107.3) or C max,ss (GMR 108.5; 90% CI 100.7, 116.9) of sitagliptin. Empagliflozin and sitagliptin were well tolerated when given alone or in combination. Five subjects (31.3%) reported at least one adverse event (AE): three (18.8%) experienced an AE while receiving empagliflozin monotherapy and three (18.8%) while receiving sitagliptin monotherapy. No adverse events were reported during the coadministration period. No AEs were regarded as drug-related by the investigator.
Conclusion
These results indicate that empagliflozin and sitagliptin can be coadministered without dose adjustments.
Similar content being viewed by others
References
Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–91.
Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32:63–71.
Kipnes M. Sodium-glucose cotransporter 2 inhibitors in the treatment of Type 2 diabetes: a review of Phase II and III trials. Clin Investig. 2011;1:145–56.
Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90.
Port A, Macha S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of BI 10773, a sodium glucose cotransporter-2 (SGLT-2), in healthy volunteers. Diabetes. 2010;59(Suppl. 1):A155.
Seman L, Macha S, Jones P, et al. Safety and tolerability of BI 10773, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, following 8-days treatment in patients with type 2 diabetes. Diabetes. 2010;59(Suppl. 1):A156.
Kim D, Wang L, Beconi M, et al. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–51.
Januvia (sitagliptin) [package insert]. 2010; Merck & Co, Inc. Whitehouse Station, NJ, USA.
Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512.
Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72.
Herman GA, Stevens C, Van DK, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–88.
Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443–53.
Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–59.
MedDRA Introductory Guide. 12th ed. Chantilly, VA: MedDRA Maintenance and Support Services Organization; 2009.
Gallwitz B. Sitagliptin: Profile of a novel DPP-4 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc). 2007;43:13–25.
Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70:2089–112.
Kania DS, Gonzalvo JD, Weber ZA. Saxagliptin: a clinical review in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33:1005–22.
Scott LJ. Linagliptin: in type 2 diabetes mellitus. Drugs. 2011;71:611–24.
Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle H-J. Pharmacokinetics and pharmacodynamics of BI 10773, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, and linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, following co-administration in healthy volunteers. Diabetes. 2011;60(Suppl. 1):A616–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
EudraCT registration number: 2008-006088-35
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Brand, T., Macha, S., Mattheus, M. et al. Pharmacokinetics of Empagliflozin, a Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor, Coadministered with Sitagliptin in Healthy Volunteers. Adv Therapy 29, 889–899 (2012). https://doi.org/10.1007/s12325-012-0055-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-012-0055-3