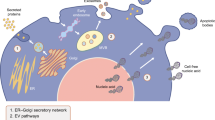
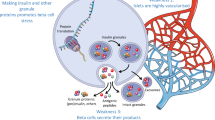
Abstract
The hallmark of type 1 diabetes (T1D) is a decline in functional β-cell mass arising as a result of autoimmunity. Immunomodulatory interventions at disease onset have resulted in partial stabilization of β-cell function, but full recovery of insulin secretion has remained elusive. Revised efforts have focused on disease prevention through interventions administered at earlier disease stages. To support this paradigm, there is a parallel effort ongoing to identify circulating biomarkers that have the potential to identify stress and death of the islet β-cells. Whereas no definitive biomarker(s) have been fully validated, several approaches hold promise that T1D can be reliably identified in the pre-symptomatic phase, such that either β-cell preservation or immunomodulatory agents might be employed in at-risk populations. This review summarizes the most promising protein- and nucleic acid-based biomarkers discovered to date and reviews the context in which they have been studied.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–13.
Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9.
Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8.
Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9.
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52.
Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diab Endocrinol. 2013;1:284–94.
Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–206.
Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–94.
Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, et al. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–9.
Maganti A, Evans-Molina C, Mirmira R. From immunobiology to β-cell biology: the changing perspective on type 1 diabetes. Islets. 2014;6:e28778.
Marré ML, James EA, Piganelli JD. β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol. 2015;3:67.
Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–74.
Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–76.
Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56:234–41.
Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med. 2013;5:211ra156.
Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–27.
Yang C, Diiorio P, Jurczyk A, O’Sullivan-Murphy B, Urano F, Bortell R. Pathological endoplasmic reticulum stress mediated by the IRE1 pathway contributes to pre-insulitic beta cell apoptosis in a virus-induced rat model of type 1 diabetes. Diabetologia. 2013;56:2638–46.
Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–20.
Liu M, Wright J, Guo H, Xiong Y, Arvan P. Proinsulin entry and transit through the endoplasmic reticulum in pancreatic beta cells. Vitam Horm. 2014;95:35–62.
Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64:2–6.
Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat. 1982;19:351–8.
Snorgaard O, Hartling SG, Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:36–42.
Watkins RA, Evans-Molina C, Terrell JK, Day KH, Guindon L, Restrepo IA, et al. Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl Res. 2016;168:96–106.e1.
Schölin A, Nyström L, Arnqvist H, Bolinder J, Björk E, Berne C, et al. Proinsulin/C-peptide ratio, glucagon and remission in new-onset type 1 diabetes mellitus in young adults. Diabet Med. 2011;28:156–61.
Røder ME, Knip M, Hartling SG, Karjalainen J, Akerblom HK, Binder C. Disproportionately elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses. The Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1994;79:1570–5.
Truyen I, De Pauw P, Jørgensen PN, Van Schravendijk C, Ubani O, Decochez K, et al. Proinsulin levels and the proinsulin:C-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia. 2005;48:2322–9.
Sims EK, Chaudhry Z, Watkins RA, Syed F, Blum J, Fangqian O, et al. Elevations in the fasting serum proinsulin:C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016. doi:10.2337/dc15-2849. Sims and colleagues demonstrated an increase in the PI:C ratio 12 months prior to T1D diagnosis in subjects followed in the TrialNet Pathway to Prevention study, with the most pronounced elevations observed in subjects ≤10 years of age. Logistic regression analysis, adjusted for age and body mass index, demonstrated an increased odds of progression to T1D with higher PI:C ratios.
Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology. 2013;154:603–8.
Kanak MA, Takita M, Shahbazov R, Lawrence MC, Chung WY, Dennison AR, et al. Evaluation of MicroRNA375 as a novel biomarker for graft damage in clinical islet transplantation. Transplantation. 2015;99:1568–73. Circulating levels of miR-375 in persons undergoing autologous or allogeneic islet transplantation were analyzed and plasma miR-375 levels were found to be significantly higher in recipients of islet transplants compared to a control group who had not undergone transplantation.
Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59:978–86.
Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen M-LM, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362.
Kim KW, Ho A, Alshabee-Akil A, Hardikar AA, Kay TWH, Rawlinson WD, et al. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes. 2016;65:996–1003.
Husseiny MI, Kaye A, Zebadua E, Kandeel F, Ferreri K. Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PLoS ONE. 2014;9:e94591.
Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A. 2016;113:E1826–34. In this article, Lehmann-Werman and colleagues introduced a cell-free insulin DNA assay based on detection of the methylation status of 6 regional CpG sites in the insulin promoter. The authors found that demethylation at all 6 sites was present in ∼80% of DNA molecules from β cells compared to less than 0.01% of DNA from other tissues. The assay was subsequently validated in samples from subjects with T1D and in persons undergoing islet transplantation.
Akirav EM, Lebastchi J, Galvan EM, Henegariu O, Akirav M, Ablamunits V, et al. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108:19018–23.
Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, et al. Elevations in circulating methylated and unmethylated preproinsulin DNA in new-onset type 1 diabetes. Diabetes. 2015;64:3867–72. Utilizing a droplet digital PCR-based assay, Fisher and colleagues demonstrated that circulating levels of both unmethylated and methylated insulin DNA were elevated in subjects with new onset T1D. Methylated insulin DNA remained elevated up to 8 weeks after T1D onset, while unmethylated insulin DNA levels decreased after diagnosis.
Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125:1163–73. Herold and colleagues analyzed samples from the TrialNet Pathway to Prevention Cohort and found that individuals at high risk for T1D had increased levels of unmethylated insulin DNA during the presymptomatic phase of T1D.
Olsen JA, Kenna LA, Spelios MG, Hessner MJ, Akirav EM. Circulating differentially methylated amylin DNA as a biomarker of β-cell loss in type 1 diabetes. PLoS ONE. 2016;11:e0152662.
Hartling SG, Knip M, Røder ME, Dinesen B, Akerblom HK, Binder C. Longitudinal study of fasting proinsulin in 148 siblings of patients with insulin-dependent diabetes mellitus. Study Group on Childhood Diabetes in Finland. Eur J Endocrinol. 1997;137:490–4.
Heding LG, Ludvigsson J. Human proinsulin in insulin-treated juvenile diabetics. Acta Paediatr Scand Suppl 1977;48–52.
Hartling SG, Lindgren F, Dahlqvist G, Persson B, Binder C. Elevated proinsulin in healthy siblings of IDDM patients independent of HLA identity. Diabetes 1989;38:1271–1274.
Lindgren FA, Hartling SG, Dahlquist GG, Binder C, Efendic S, Persson BE. Glucose-induced insulin response is reduced and proinsulin response increased in healthy siblings of type 1 diabetic patients. Diabet Med 1991;8:638–643.
Spinas GA, Snorgaard O, Hartling SG, Oberholzer M, Berger W. Elevated proinsulin levels related to islet cell antibodies in first-degree relatives of IDDM patients. Diabetes Care 1992;15:632–637.
Heaton DA, Millward BA, Gray P, Tun Y, Hales CN, Pyke DA, Leslie RD. Evidence of beta cell dysfunction which does not lead on to diabetes: a study of identical twins of insulin dependent diabetics. Br Med J (Clin Res Ed) 1987;294:145–146.
Heaton DA, Millward BA, Gray IP, Tun Y, Hales CN, Pyke DA, Leslie RD. Increased proinsulin levels as an early indicator of B-cell dysfunction in non-diabetic twins of type 1 (insulin-dependent) diabetic patients. Diabetologia 1988;31:182–184.
Lindgren FA, Hartling SG, Persson BE, Roder ME, Snellman K, Binder C, Dahlquist G. Proinsulin levels in newborn siblings of type 1 (insulin-dependent) diabetic children and their mothers. Diabetologia 1993;36:560–563.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8.
Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–62.
Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30:460–71.
Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–4.
miRBase [Internet]. [cited 2016 May 8]. Available from: http://www.mirbase.org/
Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73.
Guay C, Menoud V, Rome S, Regazzi R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal. 2015;13:17.
Lakhter AJ, Sims EK. Minireview: emerging roles for extracellular vesicles in diabetes and related metabolic disorders. Mol Endocrinol. 2015;29:1535–48.
Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–34.
Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8:e73798.
Bijkerk R, Duijs JMGJ, Khairoun M, Ter Horst CJH, van der Pol P, Mallat MJ, et al. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am J Transplant. 2015;15:1081–90.
Figliolini F, Cantaluppi V, Lena MD, Beltramo S, Romagnoli R, Salizzoni M, et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS ONE. 2014;9:e102521.
de la Torre García N, Fernández-Durango R, Gómez R, Fuentes M, Roldán-Pallarés M, Donate J, et al. Expression of angiogenic microRNAs in endothelial progenitor cells from type 1 diabetic patients with and without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:4090–8.
Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513–21.
Zampetaki A, Willeit P, Burr S, Yin X, Langley SR, Kiechl S, et al. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes. 2016;65:216–27.
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41.
van de Bunt M, Gaulton KJ, Parts L, Moran I, Johnson PR, Lindgren CM, et al. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS ONE. 2013;8:e55272.
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30.
Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–8.
Latreille M, Herrmanns K, Renwick N, Tuschl T, Malecki MT, McCarthy MI, et al. miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development. J Mol Med. 2015;93:1159–69. This article found that only a relatively small proportion (∼1%) of circulating miR-375 originates from β cells, suggesting utility of this miRNA as a biomarker of β cell death but not β cell mass.
Osipova J, Fischer D-C, Dangwal S, Volkmann I, Widera C, Schwarz K, et al. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab. 2014;99:E1661–5.
Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev. 2011;27:862–6.
Wu G-C, Pan H-F, Leng R-X, Wang D-G, Li X-P, Li X-M, et al. Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun Rev. 2015;14:798–805.
NONCODE [Internet]. [cited 2016 May 14]. Available from: http://www.noncode.org/
Hakonarson H, Grant SFA, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–4.
Plagnol V, Howson JMM, Smyth DJ, Walker N, Hafler JP, Wallace C, et al. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. 2011;7:e1002216.
Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–64.
Wallace C, Smyth DJ, Maisuria-Armer M, Walker NM, Todd JA, Clayton DG. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet. 2010;42:68–71.
Motterle A, Gattesco S, Caille D, Meda P, Regazzi R. Involvement of long non-coding RNAs in beta cell failure at the onset of type 1 diabetes in NOD mice. Diabetologia. 2015;58:1827–35.
Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S, Patel NA. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015;4:102–7.
Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37.
Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–6.
Stebbing J, Bower M, Syed N, Smith P, Yu V, Crook T. Epigenetics: an emerging technology in the diagnosis and treatment of cancer. Pharmacogenomics. 2006;7:747–57.
Grady WM, Rajput A, Lutterbaugh JD, Markowitz SD. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–2.
Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, et al. Insulin gene expression is regulated by DNA methylation. PLoS One. 2009;4:e6953.
Yang BT, Dayeh TA, Kirkpatrick CL, Taneera J, Kumar R, Groop L, et al. Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA1c levels in human pancreatic islets. Diabetologia. 2010;54:360–7.
Husseiny MI, Kuroda A, Kaye AN, Nair I, Kandeel F, Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS One. 2012;7:e47942.
Lebastchi J, Deng S, Lebastchi AH, Beshar I, Gitelman S, Willi S, et al. Immune therapy and β-cell death in type 1 diabetes. Diabetes. 2013;62:1676–80.
Fisher MM, Perez Chumbiauca CN, Mather KJ, Mirmira RG, Tersey SA. Detection of islet β-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology. 2013;154:3476–81.
Usmani-Brown S, Lebastchi J, Steck AK, Beam C, Herold KC, Ledizet M. Analysis of β-cell death in type 1 diabetes by droplet digital PCR. Endocrinology. 2014;155:3694–8.
Rui J, Deng S, Lebastchi J, Clark PL, Usmani-Brown S, Herold KC. Methylation of insulin DNA in response to proinflammatory cytokines during the progression of autoimmune diabetes in NOD mice. Diabetologia. 2016;59:1021–9. The authors showed that during progression to Type 1 diabetes, the methylation status of the insulin gene in islets from NOD mice changed in a dynamic fashion. The authors also demonstrated that treatment of isolated islets with a cocktail of pro-inflammatory cytokines led to increased methylation of the insulin gene.
Acknowledgments
This work was supported by NIH grants UC4 DK104166 (to R.G.M and C.E-M.), DK093954 (to C.E-M.), K08 DK103983 (to E.K.S), VA Merit Award I01 BX001733 (to C.E-M.), JDRF grant SRA-2014-41 (to C.E.-M.), a JDRF postdoctoral fellowship (to F.S.), and support from the Ball Brothers Foundation and the George and Frances Ball Foundation. Work in the laboratory of RGM is also supported by NIH grants R01 DK60581 and R01 DK105588. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs or the United States Government, or the JDRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Raghavendra G. Mirmira, Emily K. Sims, Farooq Syed, and Carmella Evans-Molina declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Mirmira, R.G., Sims, E.K., Syed, F. et al. Biomarkers of β-Cell Stress and Death in Type 1 Diabetes. Curr Diab Rep 16, 95 (2016). https://doi.org/10.1007/s11892-016-0783-x
Published:
DOI: https://doi.org/10.1007/s11892-016-0783-x