Abstract
Aims/hypothesis
The aim of this work was to assess whether neonatal levels of 25-hydroxyvitamin D (25(OH)D) are associated with risk of developing type 1 diabetes before the age of 18 years.
Methods
Two large-scale studies with different designs—a case-cohort and a case–control—were conducted using Danish national register data and biobank material. Weighted Cox regression and conditional logistic regression were used to calculate HRs and ORs, respectively. The concentration of 25(OH)D was assessed from neonatal dried blood spots using highly sensitive liquid chromatography–tandem mass spectrometry. Quintiles of 25(OH)D3 were used in the main analyses.
Results
The case-cohort study included 912 type 1 diabetes cases and 2866 individuals without type 1 diabetes born in Denmark between 1981 and 2002 and followed up until the end of 2012. The case–control study included 527 matched case–control pairs born between 1981 and 1999 and followed up until May 2004. Both studies found no association between 25(OH)D3 levels and later risk of developing type 1 diabetes. The neonatal total 25(OH)D levels in the studies were low: 46% (case-cohort study) and 51% (case–control study) of individuals had 25(OH)D levels <25 nmol/l.
Conclusions/interpretation
Our two large-scale national studies showed that 25(OH)D3 levels around the time of birth were not associated with later type 1 diabetes risk. Whether higher levels of 25(OH)D3 during pregnancy, acquired by higher doses of supplementation than are recommended today in most countries, could protect the offspring against type 1 diabetes cannot be ruled out by the present studies.
Similar content being viewed by others
Introduction
Type 1 diabetes is an organ-specific autoimmune disease with a complex aetiopathogenesis [1]. A worldwide annual increase in incidence has been observed over the last three decades [2]. In-depth understanding of what triggers the autoimmune response towards insulin-producing beta cells is not yet fully clarified, but both genetic and environmental factors contribute. Since changes in the proportions of high-risk HLA genes in a population are very slow (if there is any change at all), at least a part of the factors that have led to a recent increase in type 1 diabetes incidence are likely to be environmental exposures or gene–environment interactions [3].
Vitamin D is a secosteroid with an intranuclear receptor that functions as a transcription factor. The vitamin D receptor is found in most human cells including cells of the immune system and beta cells [4]. Inadequate vitamin D status is common among pregnant women and neonates [5], and in the latter may influence disease susceptibility in childhood and adolescence. Studies in mice have shown that gestational vitamin D insufficiency and deficiency increase the penetrance of type 1 diabetes [6]. Human observation and intervention studies investigating the role of vitamin D status or vitamin D supplementation during pregnancy in the risk of type 1 diabetes in offspring have so far yielded conflicting results. Only a few of these studies measured blood levels of 25-hydroxyvitamin D (25(OH)D)—a biomarker of vitamin D status [7–11].
Therefore, the aim of the present study was to examine whether low levels of 25(OH)D at birth increase the risk of developing type 1 diabetes before the age of 18 years in large-scale studies using Danish national register data. We measured 25(OH)D levels in neonatal dried blood spots (DBS) consisting of capillary blood taken from nearly all neonates in Denmark within a week after birth [12]. Here we report the results of two studies simultaneously conducted by two different research groups.
Methods
Data sources
The Danish Civil Registration System (CRS), established in 1968, registers all persons in Denmark alive on 2 April 1968 and born thereafter [13]. The unique personal identification number (CPR) from the CRS can be used for linkage with individual information from a range of other routine administrative registers and large clinical databases in the country. The Danish Childhood Diabetes Registry (DanDiabKids), established in 1996, collects information on all hospitalised incident cases of type 1 diabetes diagnosed at age 15 years and below before 1 January 1996 (i.e. born since 1981); since 2015 individuals diagnosed at up to age 18 years are included [2]. For all cases reported to the DanDiabKids, the original hospital records are scrutinised to establish the validity of the diagnosis. Statistics Denmark (SDK) maintains a large number of national registers including the National Birth Registry, the Family Register, the Population Education Register, the Income Statistics Register, the Immigration and Descendants Register, the Labor Market Register, the Danish Prevention Register and the National Patient Register [14]. Finally, the Danish Newborn Screening Biobank (DNSB) at the Statens Serum Institute (SSI) has stored neonatal DBS at −20°C (−4°F) since 1 May 1981 [12].
Assessment of vitamin D status
In both studies, vitamin D status was assessed by measuring 25(OH)D2 and 25(OH)D3 in 3.2 mm samples (also called punches) taken from DBS cards. Sample preparation and analysis was performed using liquid chromatography–mass spectrometry (LC-MS) according to a modified version of the analysis [15]. The modifications consisted of calibrations conducted by using commercially available calibrators and controls (Perkin Elmer, Waltham, MA, USA) and an online extraction step. The LC-MS system consisted of an Aria TLX2 system (Thermo Fisher Scientific, Waltham, MA, USA) with two Agilent 1100 binary pumps and two Agilent Quaternary pumps (Agilent, Santa Clara, CA, USA) connected to a Thermo TSQ Ultra triple quadrupole mass spectrometer equipped with an electrospray ion source. Online extraction was performed using a Cyclone P 0.5 mm × 50 mm Turbo flow column (Thermo Fisher Scientific) and analytical separation was achieved using Hypersil gold 50 mm × 2.1 mm, 3 μm reversed-phase column (Thermo Fisher Scientific). To control the quality of the analyses, Perkin Elmer’s MSMS Vitamin D kit for mass spectrometry was used. Two sets of controls at three different concentration levels were analysed before and after project samples within each batch. In addition, native full blood from a healthy volunteer was spotted onto filter paper and analysed before and after project samples. All the controls were set to be within 15% of the target value, otherwise the results were rejected.
The lower limit of quantification (LLOQ) was 4 nmol/l for 25(OH)D3 and 3 nmol/l for 25(OH)D2. The 25(OH)D levels from the DBS are full blood concentrations. Most 25(OH)D molecules in the bloodstream are protein bound and to approximate and report sera concentrations we corrected the original levels using the formula: serum 25(OH)D = full blood 25(OH)D × (1 / [1 − 0.61]), where 0.61 is the haematocrit fraction for capillary blood [16].
Study design, sample population and variables
Case-cohort study
The case-cohort study included a random subcohort of 3585 individuals from the CRS from among 1,360,466 individuals born in Denmark during 1981–2002, and followed up until the end of 2012; during the follow-up time, seven individuals had developed type 1 diabetes. According to power calculations, 80% power and a sample size of 1000 cases with type 1 diabetes would show a least detectable HR of 1.15 related to 1 SD difference in 25(OH)D with 60% variance explained by all the covariates [17]. As not all DBS cards can be found in the DNSB, we randomly sampled 1080 cases from the DanDiabKids among a total of 4404 in the register born in 1981–2002 and diagnosed until the end of 2012; four of the sampled cases overlapped with the seven type 1 diabetes cases from the random subcohort sample. Inclusion and exclusion criteria are shown in Fig. 1a.
For the 4661 selected individuals, information on birth outcomes, parent’s diabetes, maternal ethnicity and parent’s socioeconomic position (i.e. education, employment, income, savings) was collected from the SDK registers and linked with the information on 25(OH)D concentrations. The Danish Data Protection Agency gave permission for data retrieval and merging (J. no.: 2012-41-41156). Permission to access and analyse the DBS was granted by the Ethical Committee D of the Capital Region of Denmark (J. no.: H-3-2011-126) and the DNSB Steering Committee.
Case–control study
The nested case–control study had a 1:1 matching. Power calculation showed that a sample size of 460 matched pairs was needed to demonstrate a decreased risk of 50%; OR was 0.67 for developing type 1 diabetes. Initially, 626 matched pairs were randomly selected for 25(OH)D analysis from an already-collected material of DBS used in a study conducted by the same research group and originally comprising 2086 cases and 4172 controls [18]. In the original study, type 1 diabetes cases were included if individuals met the following criteria: (1) born between 1981 and 2002; (2) diagnosed between 1 January 1981 and 1 May 2004; (3) had a DBS card present in the DNSB. Individuals were excluded if the following criteria applied: (1) additionally diagnosed with another diabetes type; (2) insufficient material remained on the DBS card; (3) twin, triplet, etc., status (the latter represented 3% of the cohort). Controls were selected by collecting the DBS card next to the case’s card in the DNSB [18], thereby matching on date and therefore season of birth. In the randomly selected sample used in the present study, individuals were born during 1981–1999, due to the fact that < 0.5% individuals were born during 2000–2002 in the sample of the original study.
Of the 626 selected matched pairs, 527 pairs had complete information on birth outcomes and maternal age and ethnicity, collected from the SDK registers. Additionally, we had information on HLA-DQB1 genotype (HLA risk). Genotyping has been described elsewhere [18] and HLA risk categorisation is shown in Table 1. Inclusion and exclusion criteria are shown in Fig. 1b. The study was approved by the Danish Ethical Committee (H-4-2013-049) and by the DNSB Steering Committee.
Overlap of type 1 diabetes cases between case-cohort and case–control studies
Both our studies, even though conceived and conducted by two different research groups, used the same national data sources to sample study population. Consequently, 349 cases with type 1 diabetes overlapped between the case-cohort and the case–control study samples. Furthermore, all the punches from DBS cards used in the case–control study were collected already in 2007, and then stored at −20°C (−4°F) until recent 25(OH)D analysis [18]. Accordingly, the material used for the analyses in the case-cohort study was mixed in terms of sampling time: the punches from the overlapping 349 cases were collected in 2007 and the remaining punches were newly collected. While running the 25(OH)D assays, the punches of the 349 overlapping cases were distributed equally throughout all of the microtitre plates and, hence, across different 25(OH)D analysis times. This approach was used to minimise the risk of confounding between biological and batch effects [19].
Statistical analysis
As also shown previously [20], a high percentage of the 25(OH)D2 levels were below the LLOQ (3 nmol/l): 91% in the case-cohort study and 90% in the case–control study. Therefore, the main analyses reported are for 25(OH)D3 levels. Sensitivity analyses were conducted for total 25(OH)D (i.e. 25(OH)D3 + 25(OH)D2).
Case-cohort study
Descriptive data were presented as medians with interquartile range (range between first quartile and third quartile [Q1, Q3]), except for income statistics which were expressed as means (SD). Unlike the classic case-cohort design [21], only a sample of the type 1 diabetes cases from the cohort of all children born between 1981 and 2002 was included in the final analysis. Therefore the analysis was based on sampling theory: to estimate the magnitude of effect of 25(OH)D3 levels on the risk of type 1 diabetes, we used Cox regression with the cases and non-cases in the final sample weighted by their inverse sampling probabilities [22]. The standard errors of the estimated effects were computed by the robust method [23]. Sampling probabilities for individuals with and without type 1 diabetes are shown in Fig. 1a. To better capture a potential non-linear association, the 25(OH)D3 levels were divided into quintiles based on the concentrations of the random subcohort representing general population levels. The regression model was pre-defined based on literature and included the following confounders: gestational and mother’s delivery ages, birthweight, mother’s ethnicity and history of diabetes and parental socioeconomic position at the time of an individual offspring’s birth [24, 25]. Additionally, the model was adjusted for previously reported birth cohort effect in type 1 diabetes incidence increase in Denmark, expressed as a linear variable of year of birth [26]. For sensitivity reasons, analyses were conducted additionally including season in the models, coded as spring (March–May), summer (June–August), autumn (September–November) and winter (December–February), and using season-specific cut-offs for 25(OH)D3 quintiles [27]. Further sensitivity analyses were conducted in groups with 25(OH)D3 levels below and above 25 nmol/l, as well as in the groups of type 1 diabetes incidence age below and above 5 years. Possible interactions between 25(OH)D3 quintiles and sex (Wald test) were examined. Descriptive statistics was calculated in SPSS Version 22 (IBM Corporation, New York, NY, USA); regression models were run in Stata Version 14 (StataCorp, College Station, TX, USA).
Case–control study
Descriptive data were expressed as median (Q1, Q3) and the measure of effect was OR including 95% CI. Univariate and multivariate conditional logistic regression analyses were performed to examine the influence of 25(OH)D3 on odds of developing type 1 diabetes adjusted for possible confounders. 25(OH)D3 was modelled as a continuous variable and to capture a possible non-linear relationship between 25(OH)D3 levels and type 1 diabetes risk, we used quintiles based on the whole sample population. The assumptions for logistic regression were fulfilled. As in the case-cohort study, the models were based on pre-defined literature-based hypothesised associations between a confounder, 25(OH)D3 levels and type 1 diabetes risk. The following confounders were included: gestational and mother’s delivery age, birthweight and mother’s ethnicity [24, 25]. Two adjusted models were generated—one with and one without the HLA risk variable (Fig. 2). Sensitivity analyses and interactions were conducted as described under the case-cohort study. In addition, an interaction between 25(OH)D3 quintiles and HLA risk was also thought biologically plausible and was therefore examined. All analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC, USA).
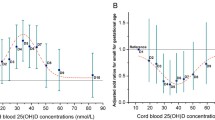
Association between the quintiles of 25(OH)D3 concentrations and type 1 diabetes risk. aQuintile values for 25(OH)D3 (nmol/l) in the case-cohort study were as follows: first quintile (Q1) 0–11.92; second quintile (Q2) 11.92–19.58; third quintile (Q3) 19.58–28; fourth quintile (Q4) 28–40.79 and fifth quintile (Q5) 40.79–130.34. Quintile values for 25(OH)D3 (nmol/l) in the case–control study were as follows: Q1 0–10.62; Q2 10.62–17.35; Q3 17.35–25.25; Q4 25.25–36.10 and Q5 36.10–117.91. bOR estimate and 95% CIs are outside range of visualisation. The shaded grey area captures the overlap of variables between the two studies
Results
In the case-cohort study, the median (Q1, Q3) for 25(OH)D3 was 23.8 (15.5, 36.7) nmol/l in individuals from the subcohort and 24.3 (14.8, 38.8) nmol/l in cases with type 1 diabetes. In the case–control study, the values were 21.1 (12.0, 32.9) nmol/l for controls and 21.3 (12.5, 33.1) nmol/l for cases. Total 25(OH)D, 25(OH)D3 and 25(OH)D2 levels did not differ between the subcohort and cases or between the controls and cases in the respective studies. Notably, 91% of 25(OH)D2 and 15% of 25(OH)D3 concentrations were below the LLOQ in the case-cohort study; respective values in the case–control study were 90% and 19%. A trend of a slight increase in 25(OH)D3 concentrations over years was observed in the case-cohort study: β (95% CI) = 0.02 (0.00, 0.04), p < 0.001 (ESM Fig. 1). All the characteristics of the sample population in both studies are presented in Table 1.
Case-cohort study
We found no association between the quintiles of 25(OH)D3 concentrations and type 1 diabetes risk either in the main adjusted analysis (Fig. 2) or in any of the sensitivity analyses (data not shown). The interaction between sex and 25(OH)D3 quintiles was not significant. Additionally, we found that gestational age (per week) (HR [95% CI] 0.96 [0.93, 1.00]), mother’s diabetes status (diabetes vs no diabetes) (1.67 [1.20, 2.33]) and ethnicity (non-western vs Danish) (0.39 [0.25, 0.61]) were associated with type 1 diabetes risk. As expected, birth cohort effect showed an increase in type 1 diabetes incidence over years (Fig. 2).
Case–control study
We found no association between 25(OH)D3 around time of birth and type 1 diabetes risk in the adjusted models, either when modelling 25(OH)D3 as a continuous variable in model 1 (excluding HLA risk: OR [95% CI] [per 25 nmol/l increase] 1.02 [0.80, 1.28]) and model 2 (including HLA risk: OR [95% CI] [per 25 nmol/l increase] 0.86 [0.60, 1.22]) or when quintiles were used (Fig. 2). Sensitivity analyses yielded essentially identical results (data not shown). Interactions between sex, HLA risk and 25(OH)D3 quintiles were not significant (data not shown). Additionally, HLA risk was significantly associated with type 1 diabetes risk. A birthweight below 2500 g was related to a decreased risk of type 1 diabetes when compared with a birthweight of 2500–4499 g, but this association was only seen in model 1 (excluding HLA risk).
Discussion
The present study examined relations between neonatal 25(OH)D3 levels and the risk of developing type 1 diabetes before the age of 18 years. We did not observe an association in either of two large-scale studies, using measured neonatal 25(OH)D3 levels and validated complete registry-based diagnosis of type 1 diabetes. While several of the previous studies in this field relied on maternal self-reported dietary intake of vitamin D, or on vitamin D supplementation during pregnancy [8, 11], only two studies so far have examined maternal serum 25(OH)D concentrations [9, 10] and one study looked at the 25(OH)D3 levels in neonatal blood from DBS [7]. Results from these three studies were conflicting: two found, like us, no support for an association [7, 9] and one found that lower maternal levels of serum 25(OH)D, measured late in the third trimester, were associated with an increased risk of developing type 1 diabetes in the offspring [10]. The three previous studies were all case–control studies. Importantly, our studies were large-scaled and population-based with limited influence of selection bias possible in the other Nordic studies [9, 10]. Similarly to the other studies, we used a validated and complete registry for type 1 diabetes diagnosis, as well as a validated and reliable 25(OH)D assay. For comparison, the basic information from all previous studies is presented in Table 2.
The neonatal screening samples that were used in our studies showed notably lower 25(OH)D concentrations compared with the levels found in other Nordic studies based on maternal serum [9, 10]. This is in accordance with the results of the studies comparing 25(OH)D3 levels in pregnant women and in cord blood [5, 28]. The Norwegian study reported a 25(OH)D maternal mean level of 41 nmol/l vs cord blood mean level of 31 nmol/l [28] and the Dutch study reported a 25(OH)D maternal mean level of 51 nmol/l vs cord blood mean level of 32 nmol/l [5]. Further, we assayed levels in DBS, while the Norwegian [10] and Finnish [9] studies assayed levels in serum. The latter has ‘traceable standards’ from the National Institute of Standards and Technology, while assays using DBS rely on a range of other methods to calibrate for accuracy, and there is no reference material or gold standard method to assess the validity of the values from DBS sample analyses [16]. Despite the differences in assay techniques, 25(OH)D concentrations from DBS assays have been shown to correlate with those using cord blood [16] and plasma [29] if the correction for haematocrit fraction is used, as was done in our studies. Thus, we have no reason to believe that the ranking of individuals with respect to neonatal 25(OH)D levels in our studies was compromised.
We also compared 25(OH)D3 concentrations in our studies with those reported by McGrath et al, measured from DBS in the Danish population-based sample comprising 848 individuals born in 1981–1994 [20]. The 25(OH)D3 concentrations reported in the study by McGrath et al were slightly higher than in our studies: median (Q1, Q3) was 32.3 (20.5, 46.6). Notably, the assays referred to by McGrath et al were run in another laboratory, where the extraction and derivatisation techniques were the same. The main difference was that McGrath et al prepared calibrators through standard addition to native whole blood, whereas our studies were based on purchased calibrators from a commercial vendor. The difference in calibrators may lead to a systematic difference in absolute levels but this, if true, would not interfere with the reliability of the results on reported associations. Moreover, while comparing the 25(OH)D3 results coming from different analysis settings, total variation in the entire analysis sample should be considered. Total variation in the analyses of our entire sample, including the samples from both studies, was 15%. Thus, the 25(OH)D3 levels in our studies differed from those reported by McGrath et al only by a few nmol/l (i.e. 25/27 nmol/l ± 15%), which is acceptable taking into account different calibration methods.
Considering biochemical aspects, the 25(OH)D concentrations reported in the Norwegian study [10] still seem to be higher than those found in our studies and in the Finnish study [9]. This corresponds with the findings from previous large-scale studies on 25(OH)D concentrations in pregnant women in Nordic countries. The Norwegian Mother and Child Cohort Study found a 25(OH)D mean level of 74 nmol/l in mid-pregnancy [30] whereas the Danish Odense Child Cohort study revealed a median mid-pregnancy level of 66 nmol/l [31]. The use of cod-liver oil by pregnant women in Norway [32] could potentially contribute to the observed relatively higher 25(OH)D levels. Consequently, the difference in findings between our study and that of Miettinen et al [9] vs Sørensen et al [10] may be due to a difference in 25(OH)D concentrations in the studied populations. This further suggests that higher levels of 25(OH)D are necessary to exert a protective effect against type 1 diabetes in a complex exposure network and that the higher levels may be potentially reached by supplementation. Similarly, Hypponen et al [33] showed that high-dose vitamin D supplementation (up to 50 μg daily) in infancy reduced type 1 diabetes risk later in life by 80%.
Despite the possibly higher 25(OH)D levels in pregnant women in Norway, type 1 diabetes incidence rates in Norway are higher than in Denmark: 23–33 per 100,000 person-years vs 20–22 per 100,000 person-years, respectively [2, 34]. Clearly, type 1 diabetes is a multifactorial disease and variations in incidence rates between different countries are caused by several factors. In the case-cohort study, maternal diabetes diagnosis, ethnicity and gestational age were associated with type 1 diabetes risk. A lower risk with increasing gestational age has been also reported previously [24]. The increased risk for those with diabetes in first-degree relatives has been reported previously as well [3]. Type 1 diabetes risk in the offspring of mothers with a non-western minority background was lower, despite lower levels of neonatal 25(OH)D. The only consistent association in the case–control study was between HLA risk group and type 1 diabetes risk, as shown previously using the same material [18].
A child with a high load of type 1 diabetes susceptibility genes may be more vulnerable to low levels of 25(OH)D than a child with a neutral or protective genotype. In the case-cohort study, to consider genetic susceptibility, we made an attempt to adjust for maternal and paternal type 1 diabetes status, which accounts for both HLA and non-HLA risk genes. Maternal diabetes status was shown to be an important predictor of type 1 diabetes hazard in the offspring but it did not change the association between disease risk and neonatal 25(OH)D3 levels. The number of individuals whose fathers had diabetes was too low to provide meaningful results. In the case–control study, adjusting for HLA risk did not change the results either and the case–control study was the first and so far the only study adjusting for HLA risk in the neonatal 25(OH)D3 and type 1 diabetes risk association. The case–control study was unable to adjust for non-HLA genetic susceptibility, which would require a very large sample size due to small effects by these numerous gene variants [3].
Interestingly, a tendency towards yearly increase in 25(OH)D3 levels was observed in the case-cohort study (ESM Fig. 1). The observed increase in 25(OH)D3 concentrations may have been caused by an increase in the number of hours of sunshine in Denmark [35] and/or by an increase in vitamin D intake through supplementation possibly due to a change in vitamin D-related awareness in the public. Additionally, decreased prevalence of smoking (negatively affecting 25(OH)D concentrations [36]) among pregnant women in Denmark may have contributed to the observed differences. On the other hand, due to the wide distribution of values throughout the years, the observed trend in 25(OH)D3 increase may be a spurious result. To take into account the possibility of 25(OH)D changes over years (and simultaneously consider increase in type 1 diabetes incidence rates over years in Denmark), all the analyses in the case-cohort study were adjusted for year of birth expressed as a linear variable.
Our studies were based on hypotheses of gestational programming, in this particular case claiming that low levels of 25(OH)D during periods of immune system maturation may cause long-term irreversible effects on immunological health [37]. It can be argued that one 25(OH)D measurement at a specific time-point (i.e. at birth) may not mirror earlier 25(OH)D pregnancy levels. Because the fetus is completely dependent on maternal 25(OH)D supply [38] and because the half-life of 25(OH)D is approximately 2–3 weeks [39], the 25(OH)D levels at birth, as a minimum, reflects fetal 25(OH)D exposure during the end of third trimester of pregnancy. At the same time, immune maturation is especially prominent with regards to development of self-tolerance from mid-pregnancy [40].
In conclusion, our two nationwide large-scale studies showed that 25(OH)D3 levels around the time of birth were not associated with type 1 diabetes risk. We cannot rule out whether higher levels of 25(OH)D3 during pregnancy, acquired by higher doses of supplementation than those recommended in Nordic countries today (10 μg daily), may protect the offspring against type 1 diabetes. Likewise, the possibility that 25(OH)D3 levels during the first and second trimesters of pregnancy could be associated with later risk of type 1 diabetes cannot be ruled out either.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- CPR:
-
Unique personal identification number
- CRS:
-
Danish Civil Registration System
- DBS:
-
Neonatal dried blood spots
- DanDiabKids:
-
Danish Childhood Diabetes Registry
- DNSB:
-
Danish Newborn Screening Biobank
- LC-MS:
-
Liquid chromatography–mass spectrometry
- LLOQ:
-
Lower limit of quantification
- Q1,Q3:
-
Interquartile range (range between first and third quartiles)
- SDK:
-
Statistics Denmark
- SSI:
-
Statens Serum Institute
References
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383:69–82
Svensson J, Lyngaae-Jorgensen A, Carstensen B, Simonsen LB, Mortensen HB (2009) Long-term trends in the incidence of type 1 diabetes in Denmark: the seasonal variation changes over time. Pediatr Diabetes 10:248–254
Groop L, Pociot F (2014) Genetics of diabetes--are we missing the genes or the disease? Mol Cell Endocrinol 382:726–739
Long MD, Sucheston-Campbell LE, Campbell MJ (2015) Vitamin D receptor and RXR in the post-genomic era. J Cell Physiol 230:758–766
Vinkhuyzen AA, Eyles DW, Burne TH et al (2015) Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: the generation R study. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2015.09.018
Giulietti A, Gysemans C, Stoffels K et al (2004) Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia 47:451–462
Cadario F, Savastio S, Pagliardini V et al (2015) Vitamin D levels at birth and risk of type 1 diabetes in childhood: a case-control study. Acta Diabetol 52:1077–1081
Granfors M, Augustin H, Ludvigsson J, Brekke HK (2015) No association between use of multivitamin supplement containing vitamin D during pregnancy and risk of type 1 diabetes in the child. Pediatr Diabetes. doi:10.1111/pedi.12334
Miettinen ME, Reinert L, Kinnunen L et al (2012) Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the offspring. Diabetologia 55:1291–1294
Sorensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC (2012) Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes 61:175–178
Stene LC, Ulriksen J, Magnus P, Joner G (2000) Use of cod liver oil during pregnancy associated with lower risk of type I diabetes in the offspring. Diabetologia 43:1093–1098
Hollegaard MV, Grauholm J, Nielsen R, Grove J, Mandrup S, Hougaard DM (2013) Archived neonatal dried blood spot samples can be used for accurate whole genome and exome-targeted next-generation sequencing. Mol Genet Metab 110:65–72
Pedersen CB (2011) The Danish civil registration system. Scand J Public Health 39:22–25
Thygesen LC, Ersboll AK (2011) Danish population-based registers for public health and health-related welfare research: introduction to the supplement. Scand J Public Health 39:8–10
Eyles D, Anderson C, Ko P et al (2009) A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin Chim Acta 403:145–151
Eyles DW, Morley R, Anderson C et al (2010) The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr Perinat Epidemiol 24:303–308
Jacobsen R, Abrahamsen B, Bauerek M et al (2013) The influence of early exposure to vitamin D for development of diseases later in life. BMC Public Health 13:515
Eising S, Svensson J, Skogstrand K et al (2007) Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: design and feasibility of an unselected case-control study. Paediatr Perinat Epidemiol 21:507–517
Leek JT, Scharpf RB, Bravo HC et al (2010) Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet 11:733–739
McGrath JJ, Eyles DW, Pedersen CB et al (2010) Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 67:889–894
Prentice RL (1986) A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 73:1–11
Gray RJ (2009) Weighted analyses for cohort sampling designs. Lifetime Data Anal 15:24–40
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the Cox model. Springer, New York
Cardwell CR, Carson DJ, Patterson CC (2005) Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood type 1 diabetes: a UK regional retrospective cohort study. Diabet Med 22:200–206
Haynes A, Bower C, Bulsara MK, Finn J, Jones TW, Davis EA (2007) Perinatal risk factors for childhood type 1 diabetes in Western Australia--a population-based study (1980-2002). Diabet Med 24:564–570
Jacobsen R, Hypponen E, Sorensen TI, Vaag AA, Heitmann BL (2015) Gestational and early infancy exposure to margarine fortified with vitamin D through a national Danish programme and the risk of type 1 diabetes: the D-Tect Study. PLoS One 10, e0128631
Wang Y, Jacobs EJ, McCullough ML et al (2009) Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin D. Am J Epidemiol 170:88–94
Godang K, Froslie KF, Henriksen T, Qvigstad E, Bollerslev J (2014) Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. Eur J Endocrinol 170:609–617
Heath AK, Williamson EJ, Ebeling PR, Kvaskoff D, Eyles DW, English DR (2014) Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J Clin Endocrinol Metab 99:3319–3324
Magnus MC, Stene LC, Haberg SE et al (2013) Prospective study of maternal mid-pregnancy 25-hydroxyvitamin D level and early childhood respiratory disorders. Paediatr Perinat Epidemiol 27:532–541
Andersen LB, Jorgensen JS, Jensen TK et al (2015) Vitamin D insufficiency is associated with increased risk of first-trimester miscarriage in the Odense Child Cohort. Am J Clin Nutr 102:633–638
Haugen M, Brantsaeter AL, Alexander J, Meltzer HM (2008) Dietary supplements contribute substantially to the total nutrient intake in pregnant Norwegian women. Ann Nutr Metab 52:272–280
Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM (2001) Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503
Skrivarhaug T, Stene LC, Drivvoll AK, Strom H, Joner G (2014) Incidence of type 1 diabetes in Norway among children aged 0-14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia 57:57–62
Cappelen J (2012) Denmark – DMI historical climate data collection 1768-2011 – with Danish abstracts. Danish Meteorological Institute, Copenhagen
Thuesen B, Husemoen L, Fenger M et al (2012) Determinants of vitamin D status in a general population of Danish adults. Bone 50:605–610
Fetahu IS, Hobaus J, Kallay E (2014) Vitamin D and the epigenome. Front Physiol 5:164
Salle BL, Glorieux FH, Delvin EE (1988) Perinatal vitamin D metabolism. Biol Neonate 54:181–187
Jones KS, Assar S, Harnpanich D et al (2014) 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab 99:3373–3381
Fadel S, Sarzotti M (2000) Cellular immune responses in neonates. Int Rev Immunol 19:173–193
Acknowledgements
We are grateful to T. I. A. Sørensen, E. Hypponen and A. A. Vaag, the members of the ‘D-tect’ project Scientific Committee and Advisory Board, for their inputs while discussing the case-cohort study, as well as statisticians K. Raymond and L. H. Ängquist from the Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, for drawing the case-cohort study samples. We would also like to express our appreciation to B. Nørgaard-Pedersen and D. M. Hougaard from the Department of Congenital Disorders, Statens Serum Institute, for their support in the initial phases of this project. Furthermore, we thank S. Eising from the Copenhagen Diabetes Research Center (CPH-DIRECT) for collecting the DBS used in the case–control study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The case-cohort study is a part of the four-year project ‘D-tect’ funded by the Programme Commission on Health, Food, and Welfare under the Danish Council for Strategic research (grant number 0603-00453B). The case–control study was supported by a scholarship from Copenhagen University and by grants from Herlev University Hospital and the Capital Region of Denmark.
Duality of interest
The authors declare that there is no conflict of interested associated with this manuscript.
Contribution statement
RJ and SUT initiated the study, participated in its design and coordination, performed the statistical analyses and helped in the drafting and editing of the article. ASC and ML performed the 25(OH)D measurements on the DBS and helped in the drafting and editing of the article. PF was the statistician for the case-cohort study, supervised the statistical analyses, and helped in the drafting and editing of the article. CBP was the statistician for the case–control study, supervised the statistical analyses, and helped in the drafting and editing of the article. FP was the principal investigator for the study from which the case–control study retained its DBS, participated in the acquisition of data, and helped in editing of the article. LCT and AA initiated the case–control study, contributed to analysis and interpretation of data, and helped in editing of the article. JS participated in the design and coordination of the study, contributed to statistical analyses, and helped in drafting and editing of the article. BLH initiated the study, participated in its design and coordination, contributed to statistical analyses, and helped in drafting and editing of the article. All authors have read and approved the final version of the manuscript. RJ and SUT are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Ramune Jacobsen and Steffen U. Thorsen are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 287 kb)
Rights and permissions
About this article
Cite this article
Jacobsen, R., Thorsen, S.U., Cohen, A.S. et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: results from two large Danish population-based studies. Diabetologia 59, 1871–1881 (2016). https://doi.org/10.1007/s00125-016-4002-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-016-4002-8