Abstract
Aims/hypothesis
The aim of this meta-analysis was to determine the relationship between HbA1c levels and subsequent cardiovascular outcomes in individuals without diabetes.
Methods
We searched Medline, Embase and Scopus from initiation of the study until the end of 2009. One reviewer searched and another verified findings. Data were extracted by one reviewer and verified by another. We accepted prospective studies in any language reporting three or more quartiles for HbA1c levels. Within quartiles, authors must have presented both numbers of patient-years at risk and cardiovascular outcomes. Outcomes per person-time at risk were regressed on average HbA1c values using Poisson regression. We pooled β coefficients using Cochran’s semi-weighted (inverse variance) random-effects model. Study quality was assessed using the Downs–Black scale.
Results
We investigated 16 datasets (nine for total cardiovascular events and seven for death) from five papers with 44,158 patients (44% men) over 404,899 patient-years of follow-up. There were 1,366 cardiovascular deaths (3.1%; 3.37/1,000 person-years) and 2,142 cardiovascular events (4.9%; 5.29/1,000 person-years). The overall meta-analytic β coefficients were 0.720 (95% CI 0.307–1.133) and 0.757 (95% CI 0.382–1.132) for cardiac death and events, respectively. Compared with the baseline value of 0.0427, an HbA1c level of 0.05 was associated with a relative risk for cardiovascular death of 1.13 (95% CI 1.05–1.21), a 0.06 value with 1.34 (95% CI 1.13–1.58), and a 0.07 HbA1c with relative risk 1.58 (95% CI 1.22–2.06). Results for total cardiovascular events were similar. The average study quality was 0.7 (70%).
Conclusions/interpretation
We conclude that HbA1c was significantly associated with cardiovascular events and deaths in persons without diabetes.
Similar content being viewed by others
Introduction
Elevated glucose levels in persons with diabetes are associated with the development of adverse cardiovascular events (CVEs) [1]. The sequence of events is presumed to be as follows [2]. In healthy persons, plasma glucose concentrations normally fluctuate within a narrow range. Aberrations in glucose metabolism in normal individuals may occur; however, fluctuations in glucose level may lead to adverse consequences. The fluctuations suspected of having the greatest consequences are postprandial glucose elevations in the form of spikes or persistent high levels. It is further proposed that glucose may have a directly toxic effect on the vascular endothelium, mediated by oxidative stress, that is independent of other cardiovascular risk factors such as hyperlipidaemia [3]. The effect may also be exerted through its substantial contribution to total glycaemic exposure as reflected by HbA1c. The oxidative stress causes microvascular damage, which eventually results in CVEs [4, 5].
A meta-analysis of randomised controlled trials explored the relationship between glycaemic control with targeted interventions compared with conventional treatments, and CVEs [6]. Different risks and outcomes were noted in people with different types of the disease. In those having type 1 diabetes, the RR of a CVE among those with glycaemic control was 0.38 (95% CI 0.26–0.56) and mainly reflected decreased cardiac and peripheral vascular events. In contrast, the RR was 0.81 (95% CI 0.73–0.91) in individuals with type 2 diabetes, and mainly reflected decreased stroke and peripheral vascular events. More importantly, the effects were more pronounced in younger persons. It has also been found that pharmacotherapeutic lowering of HbA1c levels produces a corresponding decrease in incident myocardial infarction as well as mortality [7].
In 1999, a meta-analysis [8] demonstrated that there was a relationship between postprandial glucose levels in people without diabetes and in those who had diabetes found during screening and CVEs detected on follow-up. Since that time, researchers have published reports describing a similar relationship between HbA1c and these events in persons without diabetes. To date, there have been no quantitative summaries that clearly establish that relationship. Therefore, we undertook this research to address that issue.
Methods
The model used in this research was a meta-analysis of published studies. Inclusion criteria were established a priori, defining the population of interest and acceptable studies.
Target population
The target population for this research consisted of people from the general public (population) who were screened as part of a public health programme. Participants in the screening programme must not have been diagnosed with diabetes at the time and must not have been selected for screening because of some pre-existing condition or disease (e.g. stroke, kidney failure, myocardial infarction, coronary artery disease or hypertension). All patients must have had a baseline blood HbA1c level reported as part of their screening. Even if their HbA1c level was suspicious of diabetes, they were allowed to remain in the study. Persons known to have had a diagnosis of diabetes were excluded, as were those taking hypoglycaemic drugs of any type. The exception to this was where individuals with diabetes were included in the study, but the data for those without a diagnosis of diabetes could be extracted and were adequate for analysis. Participants must have returned for at least one follow-up visit to allow for the determination of outcomes.
Study criteria
Only prospective cohorts of screened patients were allowed. Retrospective studies, such as those using databases or chart reviews as data sources, were not permitted. For studies to be eligible, they must have reported HbA1c levels in at least three quartiles. Within quartiles, they must have presented both numbers of patient-years at risk and cardiovascular outcomes. The outcomes included were acute myocardial infarction, fatal or non-fatal stroke, and cardiovascular death.
Search strategy
The databases searched included Medline, Embase and Scopus, from inception to the end of 2009. Search terms included: ‘hemoglobin/haemoglobin A1c or HbA1c’ AND ‘cardiovascular diseases’, as well as specific terms ‘stroke’, ‘myocardial infarction’, ‘death’, ‘cardiac death’ or ‘mortality’. To confine the search to one study type, we added ‘cohort or cohort study’, ‘prospective study’, or ‘follow-up’. The references from retrieved articles as well as from reviews identified in the search were manually inspected to identify further articles. One reviewer performed the search while a second verified all of the processes.
Data extraction
From each study, we obtained the following information: year in which patients were screened, number screened, range of ages or average age, percentages of men/women, reasons to exclude potential participants, time between screening and follow-up examination, number of glucose quartiles and values for those quartiles, as well as numbers of CVEs and numbers of person-years at risk in each quartile. As with the study identification, one reviewer extracted the data and another verified each data point.
Data analysis
Poisson regression models were used to assess the relationship between HbA1c levels and the RR of a cardiovascular event. The explanatory variable was the HbA1c value for the cohort, which we assumed represented a continuous distribution. When available, we used the reported mean value of each quartile, or the interval midpoint when those values were not reported. When only terminal values were presented, such as <0.045 or ≥0.065, we used those values, following the method of Coutinho et al. [8]. The dependent variable was a CVE, as previously defined. An RR equation was calculated for each CVE in each study using SAS software (SAS Institute, Cary, NC, USA), modelling the data with ‘proc genmod (distribution = Poisson)’. The haemoglobin cohort was standardised by [x* = (x − 0.0427)] such that the RR would be equal to 1 at an HbA1c of 0.0427 (i.e. equivalent to the glucose value of 0.0427 used in the original meta-analysis [8]). An option was employed within SAS to adjust for overdispersion in order to obtain robust standard errors.
We then pooled β values across studies using Cochran’s semi-weighted random-effects model [9]. To examine combinability of outcomes and other statistical issues surrounding meta-analysis, we conducted exploratory analyses such as I 2 and χ 2 to detect heterogeneity of effects [10], funnel plots [11] and regression of outcomes on sample size to detect publication bias [11].
Quality assessment
The quality of each accepted study was assessed independently by two independent reviewers using the Downs–Black scale validated for observational studies [12]. The instrument consists of 27 questions (addressing study quality of reporting, external and internal validities, bias, confounding and power), with a total possible score of 32. Scores were expressed as rates, with higher scores being better than lower scores and 1.00 being the maximum possible. Scores below 0.5 were considered ‘weak’, those between 0.50 and 0.69 were ‘fair’, from 0.70 to 0.79 were ‘good’, and those scoring between 0.81 and 1.00 were considered ‘very good’.
Results
The literature search initially identified 998 articles, which were reduced to 33 after reading titles and abstracts. After reading the full text, there were 26 potentially acceptable studies that were rejected at the final stage: 14 did not have appropriate data [1, 13–25]; four did not involve outcomes of interest [26–29]; three studied non-relevant populations [30–32]; three were duplicates [33–35]; and two were editorials/review papers [36, 37]. That left seven papers for analysis [38–44]. Figure 1 depicts the results of the literature search and disposition of the identified articles.
Table 1 summarises the characteristics of the studies that were analysed. A total of 78,015 people were screened, with 44,158 individuals (56.6%) followed over time for CVEs. Age at screening ranged from 18 to 93 years and 44% of the studied sample were men. The average length of follow-up was 9.2 years, totalling 404,899 patient-years of follow-up. The quality of the studies was 0.73, which was considered good.
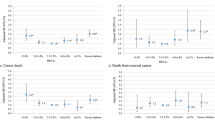
The results for each study appear in Table 2 and Fig. 2. Five articles reported cardiovascular deaths as a primary outcome [39–42, 44] in seven sets of outcome data, and two studies [42, 44] examined men and women separately. There were 1,366 cardiovascular deaths (3.1% of all persons assessed), or 3.37/1,000 person-years of follow-up. The β coefficients ranged in value from 0.172 to 1.157 and all but one were statistically significant. The overall meta-analytic β coefficient was 0.720 (95% CI 0.307–1.133). Two other studies [38, 43] also reported total cardiovascular events. There were 2,142 events (4.9% of all persons assessed), or 5.29/1,000 person-years. The β coefficients ranged from 0.172 to 2.484; again, all but one was significant. The overall meta-analytic β coefficient was 0.757 (95% CI 0.382–1.132).
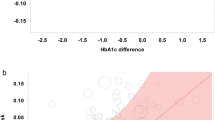
Compared with the baseline value, an HbA1c of 0.05 was associated with an RR of cardiovascular death of 1.13 (95% CI 1.05–1.21), a 0.06 HbA1c value with 1.34 (95% CI 1.13–1.58) and a 0.07 HbA1c with RR 1.58 (95% CI 1.22–2.06). Figure 3 depicts the relationship of HbA1c values and the RR of cardiovascular death, which increases exponentially with increasing HbA1c.
The funnel plots and regressions did not detect publication bias, as values were scattered equally around the meta-analytic average. Both χ 2 and I 2 did detect heterogeneity of effects (p < 0.001); however, a search for moderator variables could not identify any systematic deviations indicative of bias. Eliminating each study in turn resulted in very minor changes in outcomes, which remained significant and of the same magnitude. Elimination of studies contributing most to the heterogeneity produced a homogeneous set of four studies for each outcome, with slightly higher estimates of the RR for both of those outcomes. The heterogeneity could have arisen in part from differences in study protocols or in the measurement of HbA1c across the studies. On the other hand, as we had a priori selected a random-effects model, which tends to mitigate heterogeneity to some degree, we chose to retain all of the studies in the final model to provide a larger and broader sample.
Discussion
Cardiovascular disease (CVD) represents one of the main health problems currently facing the medical community and, consequently, healthcare systems. The number of new cases continues to rise globally, despite the investment by the pharmaceutical industry into the development of medications for treatment of CVD. Indeed, the great challenge lies with the early identification of patients at risk for CVD, concentrating on primary prevention and early treatment.
CVEs related to increased levels of HbA1c and glucose have already been thoroughly studied in people with diabetes, in studies such as the DCCT [45], UK Prospective Diabetes Study (UKPDS) [46], National Health and Nutrition Examination Survey (NHANES) [47] and Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) [48]. However, despite the increasing number of studies of glycaemic control and CVEs, few have examined that relationship in persons without diabetes [1–8]. Consequently, there has not been unequivocal evidence of such a correlation.
As cardiovascular events develop over a long period of time, it was necessary to have an adequate follow-up and sample size to appropriately study these events. Among the accepted studies for the present meta-analysis, there were data from 44,158 persons and the duration of follow-up varied from 1 year [40] to 48 years [44], with a total of 404,899 person-years. This amount of exposure appears to be adequate to study that relationship.
It was also necessary to have data from at least three quartiles of HbA1c levels in order to evaluate those events. A certain amount of precision is lost when using categories to represent groups of patients, but the large sample size offsets some of the problems. This form of regression allowed us to examine the relationship between CVEs and HbA1c in persons without diabetes over a wide range of exposure. We found that there was a progressive increase in risk with even minimally elevated HbA1c levels. That finding suggests that perhaps routine screening should begin at an early age to identify and manage emerging problems.
The combined results of the studies included in the present meta-analysis correlate HbA1c levels in people without diabetes with events and deaths associated with CVD. In Fig. 3, there can be seen a progressive rise in CVEs, which reflects a relationship with baseline levels of HbA1c in persons without diabetes. The RR for CVEs and the resulting mortality were significant in almost all studies, varying according to their baseline levels (Table 2). Overall, an approximately twofold (pooled average) risk of CVE and mortality was found in people not diagnosed with diabetes on the basis of their HbA1c value.
After our search had been completed, an important and relevant paper was published [49]. This publication reported on the Atherosclerosis Risk in Communities (ARIC) study in the USA that examined 11,092 persons over 15 years. The results were quite similar to ours in that the risk for coronary heart disease and stroke increased with higher HbA1c levels. As compared with the reference category (HbA1c 0.050–0.055), the RR for each outcome was about 1.2 for those with values between 0.055–0.060; for those with HbA1c ≥ 0.065, the RR for coronary heart disease ranged from 1.95 to 2.91, depending on the model used, and for ischaemic stroke, the RR was between 2.19 and 2.63. The paper did not report cardiovascular deaths, but only overall mortality. Therefore, the results cannot be compared with those of the present study.
The present meta-analysis has some limitations. First, the small number of accepted studies limits the extension of our results. As previously mentioned, this potential risk population has yet to be properly studied and, in the light of new studies to be conducted, the results presented here can be confirmed or revised accordingly. However, results were similar across individual studies (i.e. all but one showed significant findings) and, therefore, a trend towards an association between HbA1c and CVEs in persons without diabetes was seen regardless of the overall meta-analysis results. In addition, the individuals represented a sampling from various countries over three continents (North America, Europe and Oceania). The sample size as well as the varied population makes it possible to extrapolate data to other populations yet to be analysed, which tends to strengthen the epidemiological profile of this meta-analysis.
Second, patients with confirmed and self-reported diagnoses of diabetes were not permitted in the meta-analysis. However, HbA1c levels compatible with the current diagnostic threshold for diabetes (i.e. HbA1c >0.065) were present in five of the included studies. Thus, it is likely that there could have some patients with undiagnosed cases of diabetes and when including those patient results in the meta-analysis, an inflated risk might have been generated. The extent of such effect is unknown. Finally, the pooled data were derived from a mix of adjusted and unadjusted study results with different covariates selected for adjustments. The presence or lack of adjustments for covariates might have contributed (among other things) to the heterogeneity of effects observed in Fig. 2 , but when a homogeneous set of outcomes was used, very similar results were obtained. In addition, the majority of included studies provided sufficient statistical adjustments to major CVD risk factors and thus, the isolated effect of HbA1c could be determined. Again, since the observed effects were generally similar in both direction and value across individual study results, we believe this limitation does not significantly affect the findings of the meta-analysis.
Therefore, our findings reinforce the necessity of clarifying the point to which HbA1c levels are related to the appearance of CVD. This is exceedingly important for public health policy, as CVD prevention can have a great impact on future disease burden (morbidity and/or mortality). Future research should further investigate and possibly define lower thresholds for HbA1c values as an incremental factor in potential CVD risk.
Conclusions
Our study suggests that HbA1c, even in people who do not have diabetes, may be an indicator of metabolic alterations that could develop into CVEs, including disease-specific mortality. More studies are needed to further investigate HbA1c as a marker for CVD and validate our findings. However, it is important to highlight that normal and, more importantly, increased HbA1c levels can signal the need for prevention, intervention and follow-up in order to reduce future CVD burden.
Abbreviations
- CVD:
-
Cardiovascular disease
- CVE:
-
Cardiovascular event
References
Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (2008) Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diab Care 31:714–719
Hanefeld M, Temelkova-Kurktschiev T (2002) Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis 12:98–107
Ceriello A, Hanefeld M, Leiter L et al (2004) Postprandial glucose regulation and diabetic complications. Arch Intern Med 164:2090–2095
Ceriello A (2005) Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54:1–7
O’Keefe J, Bell D (2007) Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 100:899–904
Stettler C, Allemann S, Jüni P et al (2006) Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 152:27–38
Turnbull FM, Abraira C, Anderson RJ et al (2009) Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52:2288–2298, Erratum 52: 2470
Coutinho M, Wong Y, Gerstein H, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diab Care 22:233–240
Cochran W (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins J, Thompson S (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Downs S, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Blake D, Meigs J, Muller D, Najjar S, Andres R, Nathan D (2004) Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore longitudinal study on aging. Diabetes 53:2095–2100
Gerstein H, Pogue J, Mann J et al (2005) The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 48:1749–1755
Ko G, Chan J, Woo J et al (1998) Glycated haemoglobin and cardiovascular risk factors in Chinese subjects with normal glucose tolerance. Diabet Med 15:573–578
Lawlor D, Fraser A, Ebrahim S, Davey Smith G (2007) Independent associations of fasting insulin, glucose, and glycated haemoglobin with stroke and coronary heart disease in older women. PLoS Med 8:1396–1404
Liao D, Leonetti D, Shofer J et al (2001) Abnormal glucose tolerance and increased risk for cardiovascular disease in Japanese–Americans with normal fasting glucose. Diab Care 24:39–44
Meigs J, D’Agostino R, Nathan D, Wilson P (2002) Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diab Care 25:1845–1850
Park S, Barrett-Connor E, Wingard D, Shan J, Edelstein S (1996) GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes: the Rancho Bernardo Study. Diab Care 19:450–456
Selvin E, Coresh J, Golden S, Boland L, Brancati F, Steffes M (2005) Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes. Diab Care 28:1965–1973
Selvin E, Coresh J, Golden S, Brancati F, Folsom A, Steffes M (2005) Glycemic control and coronary heart disease risk in persons with and without diabetes: The Atherosclerosis Risk in Communities Study. Arch Intern Med 165:1910–1916
Simmons R, Sharp S, Boekholdt M et al (2008) Evaluation of the Framingham risk score in the European Prospective Investigation of Cancer-Norfolk Cohort: does adding glycated hemoglobin improve the prediction of coronary heart disease events? Arch Intern Med 168:1209–1216
Stephens J, Humphries S, Cooper J, Hurel S (2004) What are the clinical manifestations of cardiovascular disease in diabetes? Ten year analysis from a clinic based population. Br J Diab Vasc Dis 4:190–194
Stevens R, Kothari V, Adler A, Stratton I, Holman R (2001) The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci 101:671–679
Stratton I, Adler A, Neil A et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Danaei G, Lawes C, Vander Hoorn S, Murray C, Ezzati M (2006) Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet 368:1651–1659
Dilley J, Ganesan A, Deepa R et al (2007) Association of A1C with cardiovascular disease and metabolic syndrome in Asian Indians with normal glucose tolerance. Diab Care 30:1527–1532
O’Sullivan C, Hynes N, Mahendran B et al (2006) Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 32:188–197
Stakos D, Schuster D, Sparks E et al (2007) Association between glycosylated hemoglobin, left ventricular mass and aortic function in nondiabetic individuals with insulin resistance. Eur J Endocrinol 157:63–68
Chowdhury T, Lasker S (1998) Elevated glycated haemoglobin in non-diabetic patients is associated with an increased mortality. Postgrad Med J 74:480–481
Gray C, Taylor R, French J et al (1987) The prognostic value of stress hyperglycaemia and previously unrecognized diabetes in acute stroke. Diabet Med 4:237–240
Shankar A, Klein R, Klein B, Moss S (2007) Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 166:393–402
Khaw K-T, Wareham N, Luben R et al (2001) Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPICNorfolk). BMJ 22:15–18
Myint P, Sinha S, Wareham N et al (2007) Glycated hemoglobin and risk of stroke in people without known diabetes in the European Prospective Investigation Into Cancer (EPIC)-Norfolk Prospective Population Study: a threshold relationship? Stroke 38:271–275
Sigal R (2005) Hemoglobin A1c levels were associated with increased cardiovascular disease and all-cause mortality in persons with and without diabetes. ACP J Club 142:52
Gerstein H, Yusuf S (1996) Dysglycaemia and risk of cardiovascular disease. Lancet 347:949–950
Sigal RJ (2005) Haemoglobin A1c concentrations were associated with increased cardiovascular disease and all cause mortality. Evid Based Med 10:57
Adams R, Appleton S, Hill C et al (2009) Independent association of HbA1c and incident cardiovascular disease in people without diabetes. Obesity 17:559–563
Corpus R, O'Neill W, Dixon S, Timmis G, Devlin W (2003) Relation of hemoglobin A1c to rate of major adverse cardiac events in nondiabetic patients undergoing percutaneous coronary revascularization. Am J Cardiol 92:1282–1286
de Vegt F, Dekker J, Ruhe H et al (1999) Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 42:926–931
Gao L, Matthews F, Sargeant L, Brayne C (2008) An investigation of the population impact of variation in HbA1c levels in older people in England and Wales: from a population based multi-centre longitudinal study. BMC Public Health 5:54
Khaw K-T, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: The European Prospective Investigation into Cancer in Norfolk. Ann Intern Med 141:413–420
Pradhan A, Rifai N, Buring J, Ridker P (2007) HbA1c predicts diabetes but not cardiovascular disease in non-diabetic women. Am J Med 120:720–727
Singer D, Nathan D, Anderson K, Wilson P, Evans J (1992) Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes 41:202–208
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Turner RC, Millns H, Neil HA et al (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316:823–828
Muntner P, He J, Chen J, Fonseca V, Whelton PK (2004) Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III). Ann Epidemiol 14:686–695
The DECODE Study Group on behalf of the European Diabetes Epidemiology Group (2004) Prediction of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia 47:2118–2128
Selvin E, Steffes MW, Zhu H et al (2010) Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362:800–811
Duality of interest
Our study had no external funding and the authors declare no direct conflicts of interest. In the past, T. R. Einarson has directly or indirectly received funding and honoraria from NovoNordisk, Bristol-Myers Squibb, Janssen-Ortho, Eli Lilly, Generex Biotechnology, Bayer, Wyeth, Novartis, Roche, Glaxo and Lundbeck. At the time of conducting this research, M. Machado was with the Toronto Health Economics and Technology Assessment (THETA) Collaborative but is currently employed in Brazil at GlaxoSmithKline as a pharmacoeconomics manager.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos-Oliveira, R., Purdy, C., da Silva, M.P. et al. Haemoglobin A1c levels and subsequent cardiovascular disease in persons without diabetes: a meta-analysis of prospective cohorts. Diabetologia 54, 1327–1334 (2011). https://doi.org/10.1007/s00125-011-2078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2078-8