Abstract
Aims/hypothesis
People who were small at birth have an increased risk of type 2 diabetes in later life. People who were in utero during the Dutch famine had decreased glucose tolerance and raised insulin concentrations at age 50. We aimed to evaluate whether prenatal famine exposure leads to more rapid progression of impaired glucose/insulin homeostasis with increasing age.
Methods
We performed an OGTT in 702 men and women at age 50 and in 699 men and women at age 58, all born as term singletons immediately before, during or after the 1944–1945 Dutch famine.
Results
People who had been exposed to famine in utero had significantly higher 120-min glucose concentrations at age 58 compared with people who had not been exposed to famine (difference=0.4 mmol/l, 95% CI 0.1 to 0.7, adjusted for sex and BMI). Glucose tolerance deteriorated between the age of 50 and 58. The unadjusted 120-min glucose concentrations rose by 0.2 mmol/l (95% CI 0.0 to 0.4), while 120-min insulin concentrations had increased by 64 pmol/l (95% CI 48 to 82). There were no differences in the rates of glucose and insulin level increase between the famine-exposed group and the unexposed group (p=0.28 for the difference in increase in glucose concentrations and p=0.09 for insulin concentrations).
Conclusions/interpretation
Although we confirmed that undernutrition during gestation is linked to decreased glucose tolerance, the effect does not seem to become more pronounced at age 58 as compared with age 50.
Similar content being viewed by others
Introduction
People who were small at birth have an increased risk of type 2 diabetes in later life [1–7]. One interpretation of this association is that an adverse intrauterine environment permanently impairs glucose homeostasis, primarily by inducing insulin resistance [8, 9]. This interpretation is strongly supported by animal studies, which have consistently shown that experimental undernutrition during gestation leads to impaired glucose homeostasis [10]. The Dutch famine was a period of extreme food shortage in the west of the Netherlands that occurred during the last 5–6 months of World War II. The famine offers a unique opportunity to study the effects of prenatal undernutrition on health in later life. In 1998, we found the first direct evidence in humans that exposure to undernutrition during gestation reduced glucose tolerance in later life [11].
The prevalence of type 2 diabetes and IGT increases with age, mainly as a result of the increase in BMI, insulin resistance and a fall in insulin secretion [12–14]. It is not yet known whether the age-related decline in glucose tolerance is increased in people who experienced an adverse intrauterine environment. In animal experiments the effects of prenatal undernutrition on glucose tolerance have been shown to increase with age. Rats that were undernourished during gestation showed a decrease in glucose tolerance between 3 and both 12 and 15 months of age [15, 16]. In humans the prenatal influences on blood pressure have been shown to increase with age [17, 18]. The present study was designed to assess glucose tolerance in the famine cohort at age 58 and to evaluate whether the previously demonstrated effect of prenatal famine exposure on glucose tolerance in the Dutch famine birth cohort progresses with ageing.
Subjects and methods
Participants
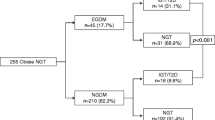
The participants were selected from the Dutch famine birth cohort (see Fig. 1). This cohort consists of 2,414 men and women born as term singletons between 1 November 1943 and 28 February 1947 in the Wilhelmina Gasthuis in Amsterdam. The selection procedures of the cohort have been described in detail elsewhere [11]. Of the cohort, 1,423 (59%) were still living in the Netherlands and their current address was known. A total of 810 people (57%) agreed to participate and underwent medical examination and a standardised interview. Two-thirds of this group (538 people, 66%) had also participated in the previous study at age 50. People who agreed to participate had mean birthweights similar to those of eligible people who did not participate (3,357 vs. 3,349 g, p=0.78). Informed consent was obtained from all participants. The local Medical Ethics Committee had approved the study. The study was carried out in accordance with the Declaration of Helsinki.
Exposure to famine
We defined the famine period based on the official daily rations for the general population older than 21 years. These rations were about 7,560 kJ/day in December 1943 and gradually decreased to about 5,880 kJ in October 1944. On 26 November 1944 the rations fell below 4,200 kJ and after 12 May 1945 they rose above 4,200 kJ again. In June 1945 rations were over 8,400 kJ. The rations during the famine did not apply to children younger than 1 year, who were relatively protected. Their official daily rations were always higher than 4,200 kJ, which is adequate according to The Oxford Nutrition Survey [19]. We considered foetuses to be exposed to famine if the average daily rations of the mother during any 13-week period of gestation were below 4,200 kJ. Babies born between 7 January 1945 and 8 December 1945 were thus exposed. We defined periods of 16 weeks each to differentiate between those who had been exposed in late gestation (born between 7 January and 28 April 1945), in mid-gestation (born between 29 April and 18 August 1945) and in early gestation (born between 19 August and 8 December 1945). Babies born before 7 January 1945 and babies conceived and born after 8 December 1945 were considered as unexposed to famine in utero and acted as a control group.
Study parameters
Birth measurements and information about the health and status of the mother were taken from the medical birth records [11]. Trained nurses carried out the medical examinations and the interview. Methods for OGTT and anthropometry at age 50 have been described in detail elsewhere [11]. At age 58, a standard 75-g OGTT was performed after an overnight fast. People with pre-existent diabetes, defined as taking oral or injected glucose-lowering medication, were excluded from the test. Plasma glucose concentrations were measured by a standardised enzymatic photometric assay on a Modular P Analyzer (Roche, Basel, Switzerland) and plasma insulin concentrations by an immunoluminometric assay on an Immulite 2000 Analyzer (Diagnostic Product Corporation, Los Angeles, CA, USA). We measured height with a fixed or portable stadiometer, weight with SECA and portable Tefal scales and waist and hip circumferences with a flexible tape measure. We interviewed all participants and asked them about their socio-economic status, their medical history, lifestyle and use of medication, using standardised questions. Current socio-economic status was coded according to ISEI-92, which is based on the person’s, or their partner’s occupation, whichever status was highest.
Statistical methods
In order to make the results comparable with those of our previous study, we defined IGT as a 120-min glucose concentration of 7.8–11.0 mmol/l and type 2 diabetes as a 120-min glucose concentration of >11.0 mmol/l. Logarithmic transformations were applied to glucose, insulin and BMI values, because they had skewed distributions. We used linear regression analysis to determine the effect of prenatal exposure to famine at different stages of gestation on glucose tolerance at age 58. To detect a possible age-related deterioration in glucose tolerance, we only included data of subjects participating at age 50 as well as at age 58, using repeated-measures analysis. We first calculated differences between the exposed and unexposed groups. Then differences were calculated between unexposed subjects and subjects prenatally exposed to famine in late, mid- or early gestation. We adjusted for maternal and birth characteristics, sex, BMI, smoking and socio-economic status. For the last three variables, measurements at age 50 and age 58 were both included in the repeated-measures analysis.
Results
Study group characteristics
We were able to perform a standard OGTT on 699 of the 810 people (86%) asked to participate in the study at age 58. Seventy of the 810 people had to be excluded from the OGTT, because they had pre-existing diabetes (see Table 1 for distribution over study groups). The test was not performed on another 41 people because they had not adhered to fasting instructions (n=7) or because of difficulties in venepuncture (n=34). For 446 of these 699 people, data on 120-min glucose and insulin values at age 50 were available. Of the 699 participants, 291 (42%) had been exposed to famine in utero. The response rates were similar in the famine-exposed and control groups. Table 1 shows that mothers exposed in late and mid-gestation weighed significantly less at the last prenatal visit than mothers who were unexposed to famine. Mothers exposed during late gestation gained almost no weight in the last trimester of pregnancy. Babies exposed to famine in late and mid-gestation were lighter and shorter than unexposed babies. They also had smaller heads and placentas.
Famine exposure and plasma glucose and insulin concentrations at age 58
People who had been exposed to famine in utero had significantly higher plasma glucose concentrations 120 min after administration of the standard oral glucose load than unexposed people (Table 2). After adjusting for sex and BMI, 120-min glucose concentrations were 0.4 mmol/l (95% CI 0.1 to 0.7) higher among the exposed people than among unexposed people. For people exposed during late gestation the difference compared with unexposed people was 0.3 mmol/l (95% CI −0.1 to 0.8), for people exposed during mid-gestation 0.4 mmol/l (95% CI 0.0 to 0.9) and for people exposed during early gestation 0.4 mmol/l (95% CI −0.1 to 1.0). After adjustment for sex and BMI, 120-min insulin concentrations were 27 pmol/l (95% CI 0 to 58) higher among exposed than among unexposed people. The difference for people exposed during late gestation compared with unexposed people was 25 pmol/l (95% CI −12 to 68). The difference for people exposed during mid-gestation was 22 pmol/l (95% CI −18 to 68) and for people exposed during early gestation 37 pmol/l (95% CI −12 to 97).
Effects of mother’s weight and birthweight
The weight of the mother at the last prenatal visit, birthweight, birth length and head circumference were all inversely related to 120-min glucose and insulin concentrations. Adjusted for sex and BMI the 120-min glucose concentrations increased by 0.8% (95% CI 0.5 to 1.1) per kilogram decrease in mother’s last weight, 13.2% (95% CI 7.4 to 19.2) with each kilogram decrease in birthweight, 2.6% (95% CI 1.4 to 3.9) with each centimetre decrease in birth length and 1.9% (95% CI 0.3 to 3.7) with each centimetre decrease in head circumference, all adjusted for sex and adult BMI. The 120-min insulin concentrations increased by 17 pmol/l (95% CI 10 to 23) per kilogram decrease in the mother’s last weight. Additional adjusting for birthweight showed that the effect of prenatal exposure to famine on 120-min glucose concentrations was larger than could be explained by the famine-related differences in birthweight. After adjustment for sex, BMI and birthweight, 120-min glucose concentrations were 0.2 mmol/l (95% CI −0.1 to 0.6) higher among the exposed than among unexposed people. Adjusting for other possible confounding variables including maternal age at delivery, current smoking status, current socio-economic status or alcohol consumption had little effect on the association between famine exposure and 120-min glucose concentrations. The prevalence of IGT and type 2 diabetes based on the OGTT did not differ significantly between the exposed and the unexposed people.
Effects of age
Table 3 shows the effects of age on progression of 120-min glucose and insulin concentrations in people who participated at age 50 as well as at age 58. Mean glucose concentrations after 120 min had increased by 0.2 mmol/l (95% CI 0.0 to 0.4). Mean insulin concentrations after 120 min had increased by 64 pmol/l (95% CI 48 to 82). A large part of the increase in 120-min glucose and insulin concentrations could be attributed to an increase in BMI. Mean BMI had increased by 1.1 units (95% CI 0.9 to 1.3). Adjusted for sex and BMI at both ages, the 120-min glucose concentrations between age 50 and 58 had increased non-significantly by 0.1 mmol/l (95% CI −0.1 to 0.2), while the adjusted 120-min insulin concentrations had increased by 51 pmol/l (95% CI 38 to 64). Exposure to famine in utero was not associated with an excess in age-related glucose tolerance deterioration (0.0 vs. 0.1 mmol/l for exposed vs. unexposed, p=0.28), nor with an excess of age-related rise in insulin (39 vs. 58 pmol/l for exposed vs. unexposed, p=0.09), nor an excess of age-related rise in BMI (1.0 vs. 1.2 unit for exposed vs. unexposed, p=0.48) (all adjusted for sex and BMI). Table 3 shows that the relatively largest increases in plasma glucose and insulin concentrations occurred among people who were born before the famine compared with people conceived after the famine. The mean plasma glucose was 0.4 mmol/l higher (p=0.04) and the mean plasma insulin was 24 pmol/l higher (p=0.08). There was a small effect of birthweight on the increase in 120-min glucose concentrations between the age of 50 and 58. For each kilogram decrease in birthweight, there was a 6% (95% CI 0 to 12) larger increase in 120-min glucose concentrations, adjusted for sex and BMI at both ages. Birthweight did not affect the increase in 120-min insulin concentrations.
Discussion
In this unique cohort study of subjects born before, during and after the Dutch famine, we found that undernutrition during gestation was associated with reduced glucose tolerance and raised insulin concentrations at age 58. This confirms our previous findings at age 50 [11]. We found that both 120-min glucose and insulin concentrations were higher in people exposed to famine at any stage of foetal development than in unexposed people. Importantly, this effect was larger than could be explained by the lower birthweight of babies born during the famine and by the low weight gain of their mothers. As with our previous study, this association was independent of people’s current BMI [11].
This is a population-based study of men and women recruited from the original famine cohort and still living in the Netherlands. At this age a significant number of individuals have overt type 2 diabetes and related diseases, especially cardiovascular disease. Selective participation of the more healthy subjects in our cohort could have influenced our results. Although we maximised response rates by visiting and studying subjects in their homes if necessary, people with type 2 diabetes, cardiovascular disease, high cholesterol levels and high blood pressure were less likely to attend the survey clinic (data not shown). However, there was no evidence of a difference in response rates between those who were famine-exposed or non-exposed. Consequently, we do not think that selective participation has affected the relative differences in glucose and insulin levels among the exposed and the unexposed groups. It could, however, have affected our power to detect effects of famine on type 2 diabetes.
Glucose tolerance deteriorated between the age of 50 and 58. A large part of this decline could be attributed to an increase in BMI. The decline in glucose tolerance was not more marked in the famine-exposed groups than in the control groups (Table 3). People who were born before the famine had the relatively largest increase in 120-min glucose and insulin concentrations. A possible explanation is that these people experienced the famine during their first year of life, which may have affected their postnatal growth pattern. Reduced early growth has been shown to be associated with later IGT and type 2 diabetes [20, 21]. Although infants were relatively protected during the famine, their official daily rations always being higher than 4,200 kJ, we do not know whether the famine affected their growth [19]. Early mortality rates were highest for people born before the famine and the deaths were mainly related to undernutrition or infections, indicating that growth was probably also affected [22].
We found that people with low birthweight had a greater age-related progression of glucose intolerance. A larger age-related progression of raised blood pressure has already been demonstrated for people with low birthweight, but to the best of our knowledge this is the first direct evidence of an age-related amplification of the effect of reduced foetal growth on glucose tolerance in humans [17, 18].
The effect of prenatal exposure to famine on glucose tolerance could be mediated through a number of mechanisms. Animal experiments indicate that undernutrition during gestation affects the development of the pancreas, which leads to an impaired function of the beta cell and consequently insulin deficiency [23]. In contrast, most human evidence points to the importance of insulin resistance. People who were thin at birth or had low birthweights were shown to be more insulin resistant than adults [24, 25]. The raised insulin concentrations that we found in famine-exposed individuals (Table 2) are consistent with insulin resistance acting as a mediator of the effect of famine exposure on glucose tolerance. Increasing evidence suggests that skeletal muscle is a key site for programming of insulin resistance. Muscle is a major site of glucose uptake and associations between a low ponderal index and altered metabolism of adult skeletal muscle have been found [26, 27]. Recently, an association between low birthweight and specific changes in muscle insulin-signalling protein expression was found [28]. Another mechanism that could explain the association between prenatal exposure to famine and later glucose intolerance involves the hypothalamic-pituitary-adrenal (HPA) axis. Maternal sheep and guinea pigs that were undernourished in late gestation gave birth to offspring in which HPA function was altered in adult life [29, 30]. In humans, low birthweight is associated with elevated basal plasma cortisol concentrations and increased adrenocortical responsiveness to adrenocorticotrophin at adult age [31, 32]. It has been hypothesised that undernutrition during gestation alters the setpoint of the HPA axis resulting in an increased activity and consequently an increased secretion of glucocorticoids, which is associated with glucose intolerance and insulin resistance [33, 34].
In summary, although we confirmed that poor nutrition in utero is related to decreased glucose tolerance in later life, the effect of prenatal exposure to the Dutch famine does not seem to become more pronounced at age 58 as compared with age 50. Progression of glucose intolerance was found to be related to famine exposure during the first year of life and to low birthweight. This suggests that prenatal famine exposure, famine exposure during the first year of life and low birthweight may contribute to glucose intolerance by different mediating mechanisms.
Abbreviations
- HPA:
-
hypothalamic-pituitary-adrenal
References
Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD (1991) Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022
Phipps K, Barker DJP, Hales CN, Fall CHD, Osmond C, Clark PMS (1993) Fetal growth and impaired glucose tolerance in men and women. Diabetologia 36:225–228
Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP (1994) Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 37:624–631
Fall CHD, Osmond C, Barker DJP et al (1995) Fetal and infant growth and cardiovascular risk factors in women. BMJ 310:428–432
Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ (1996) Birth weight and adult hypertension, diabetes mellitus and obesity in US men Circulation 94:3246–3250
Fall CHD, Stein CD, Kumaran K, Cox V, Osmond C, Barker DJP (1998) Size at birth, maternal weight, type 2 diabetes mellitus in South India. Diabet Med 15:220–227
Mi J, Law C, Zhang K, Osmond C, Stein C, Barker DJP (2000) Effects of infant birth weight and maternal body mass index in pregnancy and components of the insulin resistance syndrome. Ann Intern Med 4:253–260
Barker DJP (1998) Mothers, babies and health in later life. Churchill Livingstone, Edinburgh
Phillips DIW (1996) Insulin resistance as a programmed response to fetal undernutrition. Diabetologia 39:1119–1122
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633
Ravelli ACJ, van der Meulen JHP, Michels RPJ et al (1998) Glucose tolerance in adults after prenatal exposure to famine. Lancet 351:173–177
Harris MI, Wilbur C, Knowler WC, Bennet PH (1987) Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20–74 yr. Diabetes 36:523–534
Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U (1996) Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes 45:947–953
Basu R, Breda E, Oberg AL, Powell CC et al (2003) Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52:1738–1748
Hales CN, Desai M, Ozanne SE, Crowther NJ (1996) Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans 24:341–350
Garofano A, Czernichow P, Breant B (1999) Effect of ageing on beta-cell mass and function in rats malnourished during the perinatal period. Diabetologia 42:711–718
Law CM, Swiet de M, Osmond C et al (1993) Initiation of hypertension in utero and its amplification throughout life. Br J Obstet Gynaecol 306:24–27
Hardy R, Kuh D, Langenberg C, Wadsworth MEJ (2003) Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet 362:1178–1183
Burger GCE, Sandstead HR, Drummond JC (1948) Malnutrition and starvation in Western Netherlands, September 1944 to July 1945. Part I and II. General State Printing Office, The Hague
Eriksson JG, Forsen TJ, Osmond C, Barker DJ (2003) Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 26:3006–3010
Barker DJ (2004) The developmental origins of chronic adult disease. Acta Paediatr Suppl 93:26–33
Roseboom TJ, van der Meulen JHP, Osmond C, Barker DJP, Ravelli ACJ, Bleker OP (2001) Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol 15:220–225
Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ (1991) Islet function in offspring of mothers on low-protein diet during gestation. Diabetes 40:115–120
Phillips DIW, Barker DJP, Hales CN, Hirst S, Osmond C (1994) Thinness at birth and insulin resistance in adult life. Diabetologia 37:150–154
McKeigue PM, Lithell HO, Leon DA (1998) Glucose tolerance and resistance to insulin-stimulated glucose uptake in men aged 70 years in relation to size at birth. Diabetologia 41:1133–1138
Shulman G, Rothman D, Jue T, Stein P, deFronzo R, Shulman R (1990) Quantitation of muscle glycogen synthesis in normal subjects with non-insulin dependent diabetes by C nuclear magnetic resonance spectrography. N Engl J Med 322:223–228
Taylor DJ, Thompson CH, Kemp GJ et al (1995) A relationship between impaired fetal growth and reduced muscle glycolysis revealed by 31P magnetic resonance spectroscopy. Diabetologia 38:1205–1212
Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA (2005) Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48:547–552
Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JRG (2003) Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology 144:2933–2940
Lingas RI, Matthews SG (2001) A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology 73:302–311
Phillips D, Walker B, Reynolds R et al (2000) Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35:1301–1306
Reynolds R, Walker B, Syddall H et al (2001) Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab 86:245–250
Levitt N, Lambert E, Woods D, Hales C, Andrew R, Seckl J (2000) Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J Clin Endocrinol Metab 85:4611–4618
Rizza RA, Mandarino LJ, Gerich JE (1982) Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 54:131–138
Acknowledgements
This study was funded by the Netherlands Heart Foundation (Grant Number 2001B087), the Academic Medical Centre (Amsterdam, the Netherlands) and the Medical Research Council (UK). We thank the participants for their willing cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Rooij, S.R., Painter, R.C., Roseboom, T.J. et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia 49, 637–643 (2006). https://doi.org/10.1007/s00125-005-0136-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-0136-9